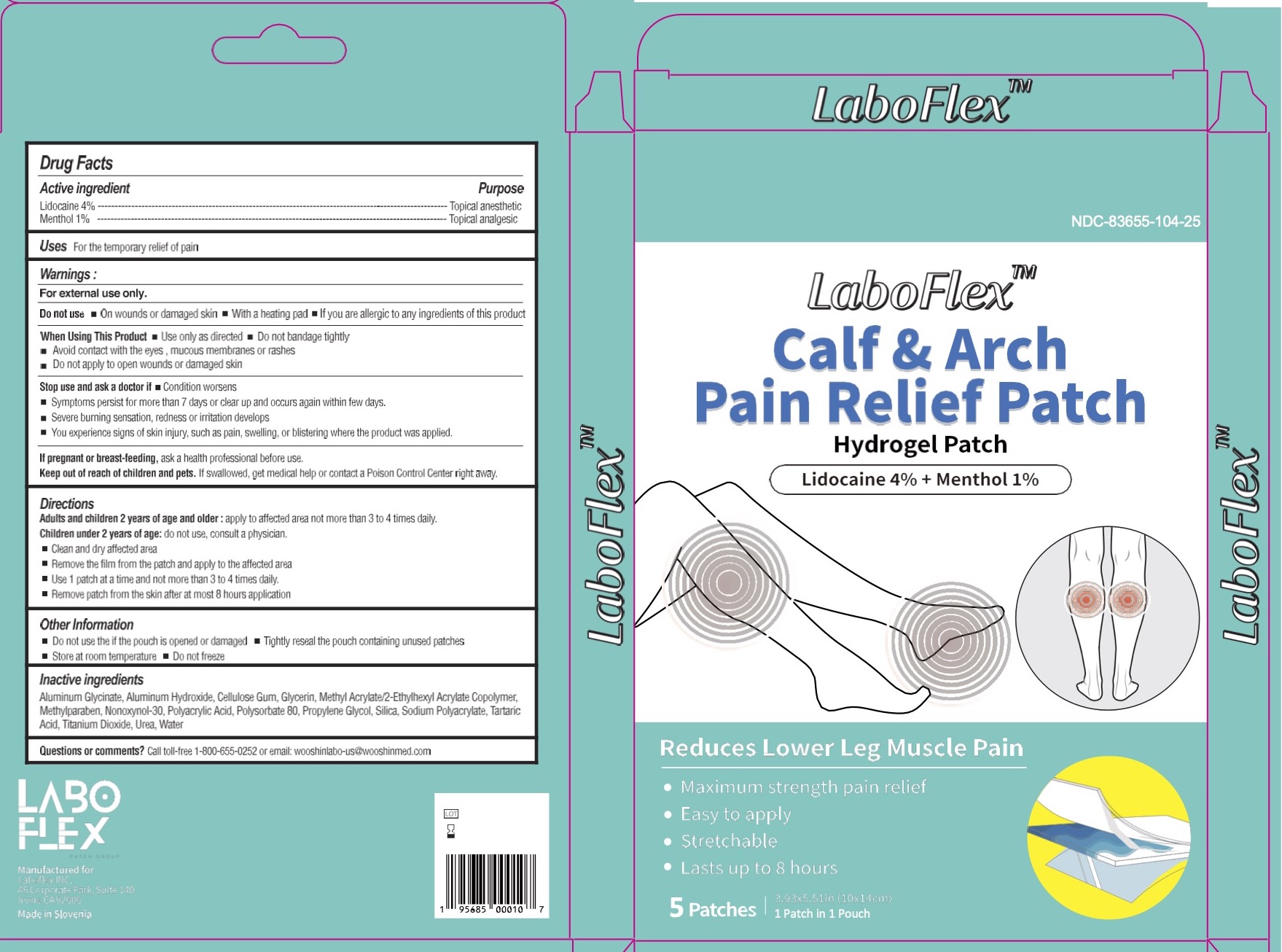
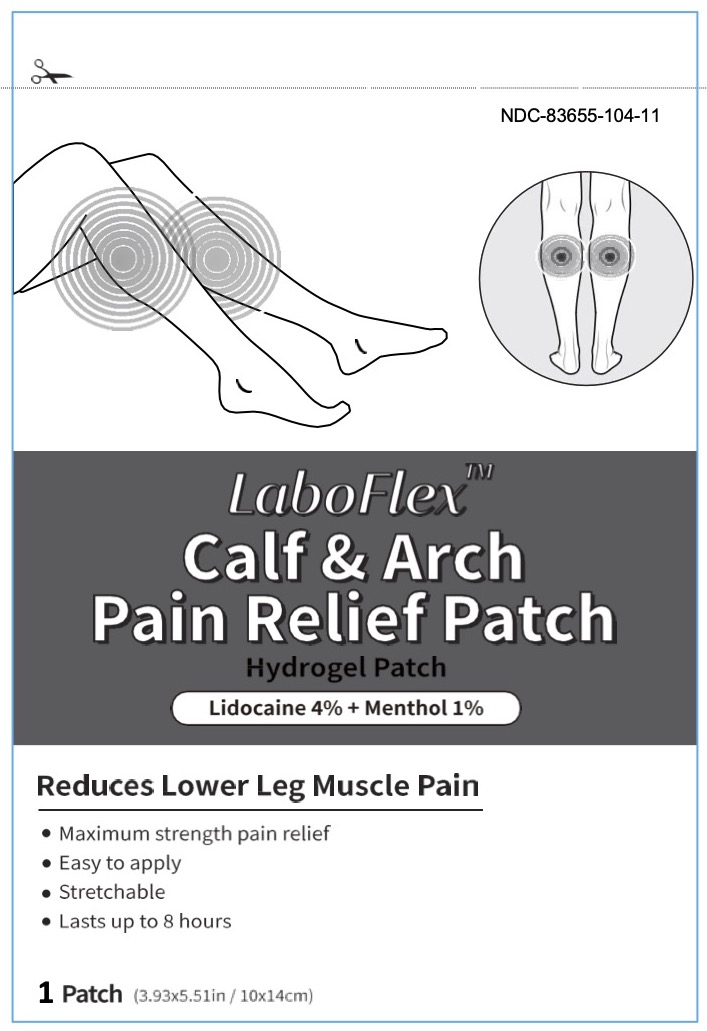
LABOFLEX Calf & Arch Pain Relief Patch - lidocaine 4% and menthol 1%
LABOFLEX Calf and Arch Pain Relief by
Drug Labeling and Warnings
LABOFLEX Calf and Arch Pain Relief by is a Otc medication manufactured, distributed, or labeled by Laboflex, Inc., WOOSHIN LAPACHE d.o.o.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LABOFLEX CALF AND ARCH PAIN RELIEF- lidocaine 4% and menthol 1% patch
Laboflex, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LABOFLEX Calf & Arch Pain Relief Patch - lidocaine 4% and menthol 1%
Inactive ingredients
Aluminum Glycinate, Aluminum Hydroxide, Cellulose Gum, Glycerin, Methyl Acrylate,/2-Ethylhexyl Acrylate Copolymer, Methylparaben, Nonoxynol-30, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, Silica, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Urea, Water
Directions:
Adults and children 2 years of age and older: : apply to affecetd area not more than 3 to 4 times daily.
Children under 2 years of age: do not use, consult a physiiacn.
- clean and dry affected area
- remove the film from the patch and apply to the affected area
- Use 1 patch at a time and not more than 3 to 4 times daily.
- Remove patch from the skin after at most 8 hours application
Keep Out of Reach of Children
Keep Out of Reach of Children
- If swallowed, get medical help or contact a Poison Control Center right away.
Stop Use and Ask a Doctor if
Stop use and ask doctor if:
- Condition worsens
- Symptoms persist for more than 7 days or clear up and occur again within a few days
- Severe burning sensation, redness or irritation developss
- You experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
When Using This Product
When using the product:
- use only as directed.
- do not bandage tightly
- Avoid contact with the eyes, mucous membranes or rashes
- do not apply to open wounds or damaged skin
Do not Use
Do not use:
- On wounds or damaged skin
- With a heantig pad
- If you are allergic to any ingredients of this product
| LABOFLEX CALF AND ARCH PAIN RELIEF
lidocaine 4% and menthol 1% patch |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Laboflex, Inc. (128394050) |
| Registrant - Laboflex, Inc. (128394050) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| WOOSHIN LAPACHE d.o.o. | 507385209 | manufacture(83655-104) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.