EPURIS- isotretinoin capsule
Epuris by
Drug Labeling and Warnings
Epuris by is a Prescription medication manufactured, distributed, or labeled by Galephar Pharmaceutical Research Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
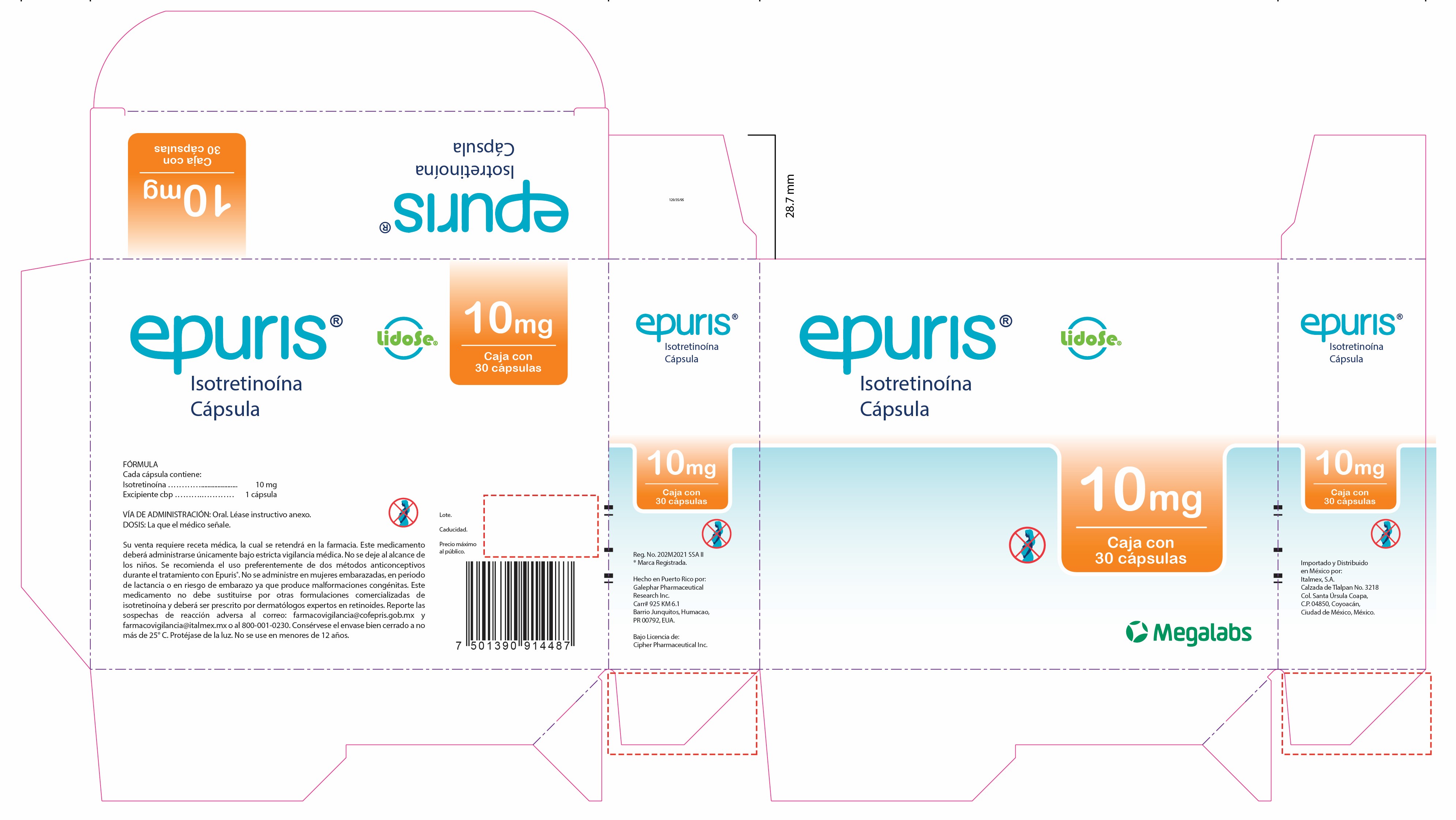
- Epuris 10 mg Box of 3 blister cards
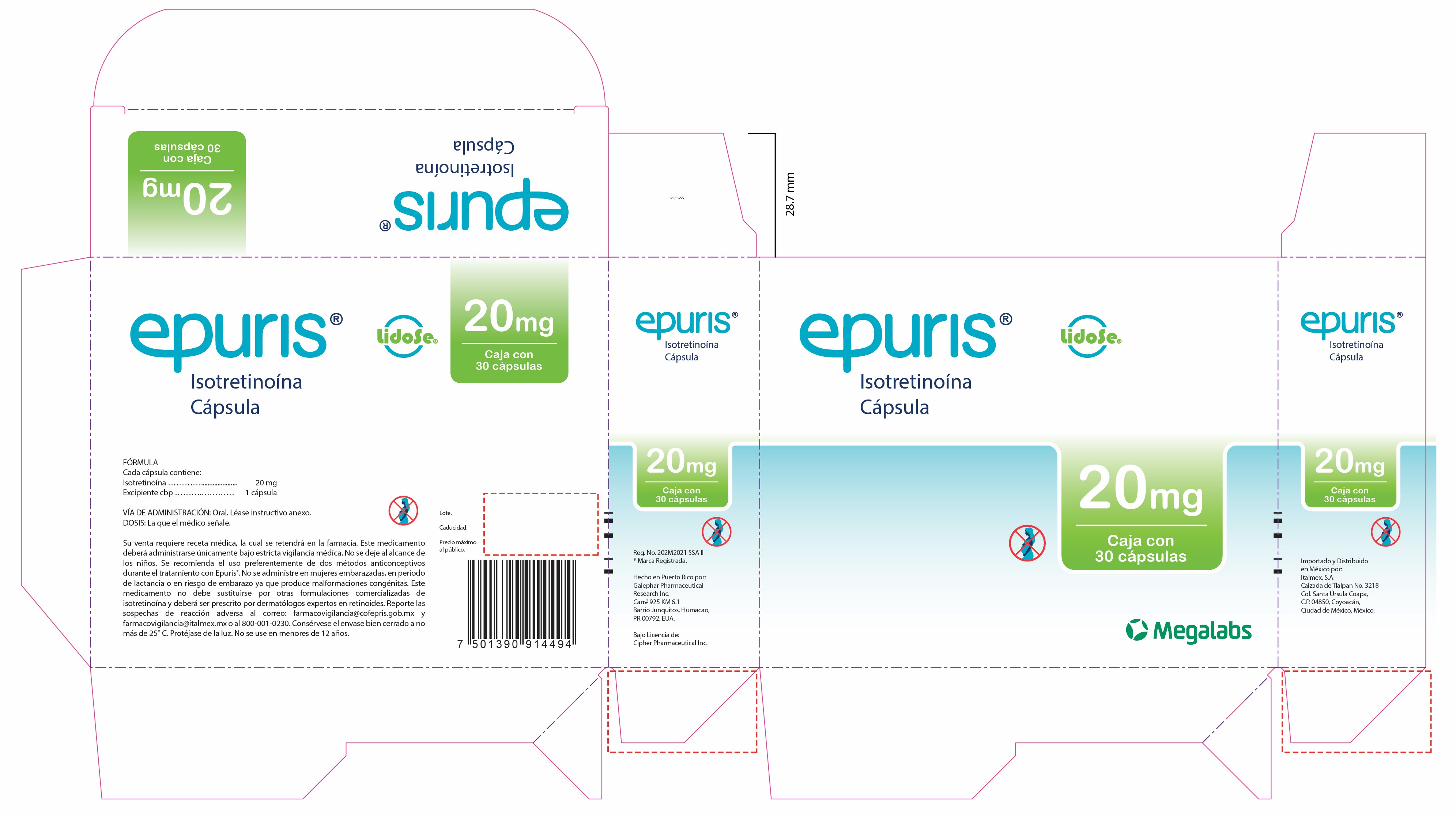
- Epuris 20 mg Box of 3 blister cards
-
INGREDIENTS AND APPEARANCE
EPURIS
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66277-335 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 20 mg Inactive Ingredients Ingredient Name Strength PROPYL GALLATE (UNII: 8D4SNN7V92) SOYBEAN OIL (UNII: 241ATL177A) PEG-32 HYDROGENATED PALM GLYCERIDES (UNII: G6EP177239) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Product Characteristics Color red Score no score Shape CAPSULE Size 22mm Flavor Imprint Code G241;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66277-335-30 3 in 1 BOX 10/01/2023 1 NDC: 66277-335-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 10/01/2023 EPURIS
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66277-336 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 10 mg Inactive Ingredients Ingredient Name Strength PROPYL GALLATE (UNII: 8D4SNN7V92) SOYBEAN OIL (UNII: 241ATL177A) PEG-32 HYDROGENATED PALM GLYCERIDES (UNII: G6EP177239) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Product Characteristics Color yellow Score no score Shape CAPSULE Size 18mm Flavor Imprint Code G240;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66277-336-30 3 in 1 BOX 10/01/2023 1 NDC: 66277-336-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 10/01/2023 Labeler - Galephar Pharmaceutical Research Inc. (003551624) Registrant - Galephar Pharmaceutical Research Inc. (968996160)
Trademark Results [Epuris]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EPURIS 98465736 not registered Live/Pending |
Atecom LLC 2024-03-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.