HPR Emollient Foam by PruGen, Inc. HPR EMOLLIENT FOAM-
HPR Emollient Foam by
Drug Labeling and Warnings
HPR Emollient Foam by is a Other medication manufactured, distributed, or labeled by PruGen, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
INDICATIONS FOR USE
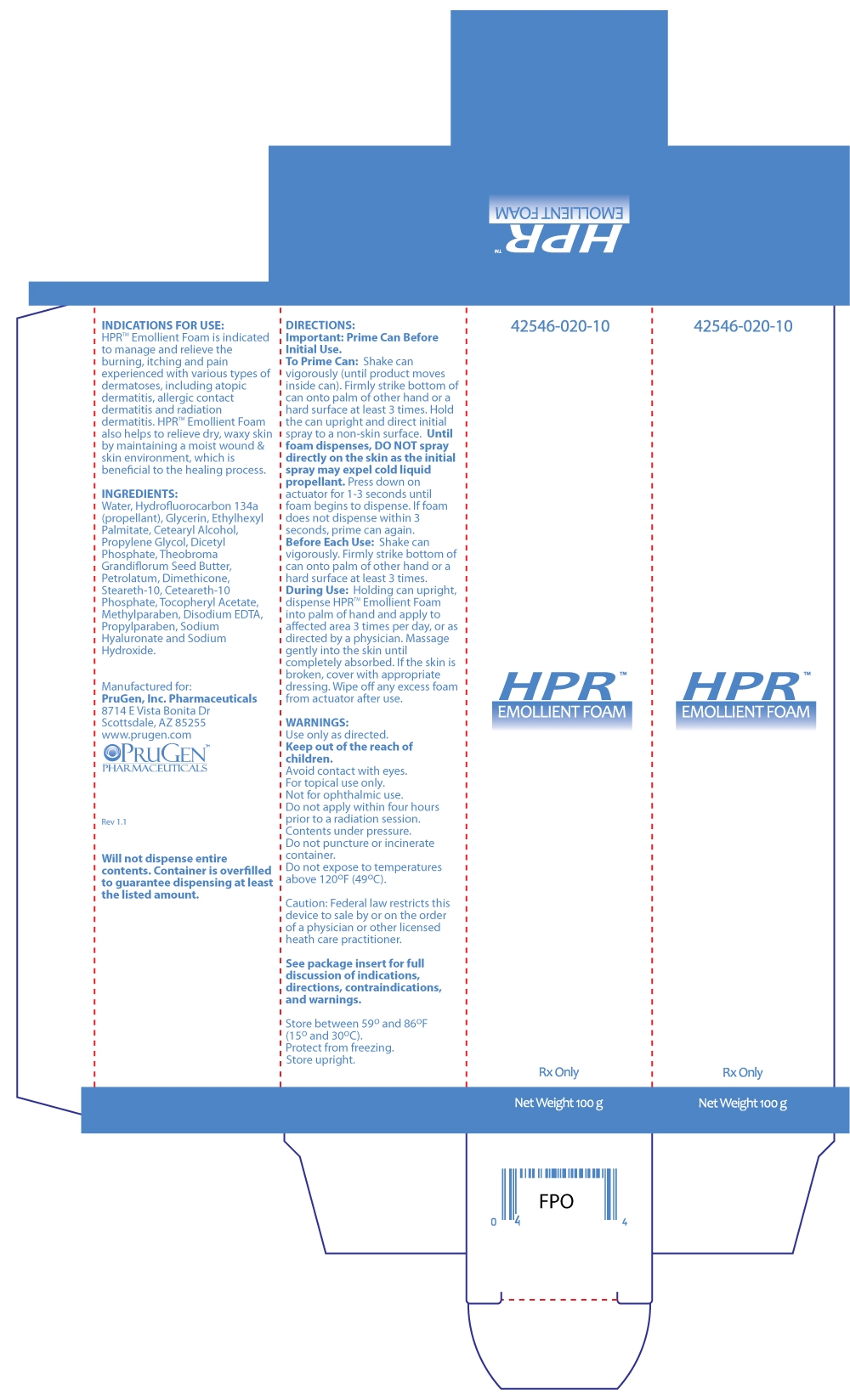
HPR Emollient Foam is indicated to manage and relieve the burning, itching and pain experienced with various types of dermatoses, including atopic dermatitis, allergic contact dermatitis and radiation dermatitis. HPR Emollient Foam also helps to relieve dry, waxy skin by maintaining a moist wound & skin environment, which is beneficial to the healing process.
- CONTRAINDICATIONS
-
WARNINGS
Use only as directed.
Keep out of the reach of children.
Avoid contact with eyes.
For topical use only.
Not for ophthalmic use.
Do not apply within four hours prior to a radiation session.
Contents under pressure.
Do not puncture or incinerate container.
Do not expose to temperatures above 120oF (49oC).
-
PRECAUTIONS AND OBSERVATIONS
HPR Emollient Foam does not contain a sunscreen and should not be used prior to extended exposure to the sun.
If clinical signs of infection are present, appropriate treatment should be initiated; use of HPR Emollient Foam may be continued during the anti-infective therapy.
If the condition does not improve within 10-14 days, consult a physician.
HPR Emollient Foam may dissolve fuchsin when this dye is used to define the margins of the radiation fields to be treated.
-
DIRECTIONS
Important: Prime Can Before Initial Use.
To Prime Can: Shake can vigorously (until product moves inside can). Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times. Hold the can upright and direct initial spray to a non-skin surface. Until foam dispenses, DO NOT spray directly on the skin as the initial spray may expel cold liquid propellant. Press down on actuator for 1-3 seconds until foam begins to dispense. If foam does not dispense within 3 seconds, prime can again.
Before Each Use: Shake can vigorously. Firmly strike bottom of can onto palm of other hand or a hard surface at least 3 times.
During Use: Holding can upright, dispense HPRTM Emollient Foam into palm of hand and apply to affected area 3 times per day, or as directed by a physician. Massage gently into the skin until completely absorbed. If the skin is broken, cover with appropriate dressing. Wipe off any excess foam from actuator after use.
-
INGREDIENTS
Water, Hydrofluorocarbon 134a (propellant), Glycerin, Ethylhexyl Palmitate, Cetearyl Alcohol, Propylene Glycol, Dicetyl Phosphate, Theobroma Grandiflorum Seed Butter, Petrolatum, Dimethicone, Steareth-10, Ceteareth-10 Phosphate, Tocopheryl Acetate, Methylparaben, Disodium EDTA, Propylparaben, Sodium Hyaluronate and Sodium Hydroxide.
-
HOW SUPPLIED
HPR Emollient Foam is available in a 100 g canister (NDC: 42546-020-10).
Will not dispense entire contents. Container is overfilled to guarantee dispensing at least the listed amount.
Caution: Federal law restricts this device to sale by or on the order of a physician or other licensed heath care practitioner.
Store between 59o and 86oF (15o and 30oC). Protect from freezing. Store upright.
Manufactured for:
PruGen, Inc. Pharmaceuticals
8714 E Vista Bonita Dr
Scottsdale, AZ 85255
www.prugen.com
-
PR Cream Box Label
NDC: 42546-135-04
PR cream Nonsteroidal Cream
For Topical Dermatological Use Only
Dispense as a complete kit
Kit includes:
-2 tubes PR cream - 2oz. (56.7g)
-2 tubes PruDrate moisturizing cream - 2oz. (56.7g)
- Patient Instructions
Rx Only
PruGen, Inc. Pharmaceuticals
-
INGREDIENTS AND APPEARANCE
HPR EMOLLIENT FOAM
dressing, wound, drugProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:42546-020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:42546-020-10 1 in 1 BOX 1 100 g in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K113772 02/01/2012 Labeler - PruGen, Inc. (929922750)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.