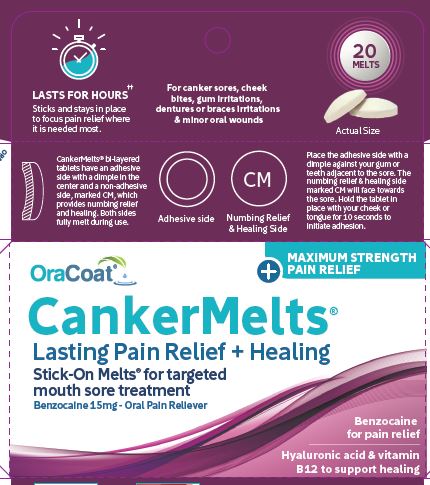
CANKERMELTS- benzocaine lozenge
CankerMelts by
Drug Labeling and Warnings
CankerMelts by is a Otc medication manufactured, distributed, or labeled by Quest Products, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient (in each self-adhering tablet)
- Purpose
- Uses
-
Warnings
Methemoglobinemea warning:
Use of this product may cause methemoglobinemea, a serious- condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert:
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
Do not use:
- this product for more than 7 days unless directed by a doctor or dentist
- in children under 5 years of age
-
Directions
Adults and children age 5 and older:
Affix one tablet as directed below and allow to slowly dissolve in the mouth. May be repeated every 2 hours as needed or directed by a doctor or dentist. This product may last over 2 hours.
Placement:
CankerMelts are bi-layered tablets with a dimple on the adhesive side and a pain reliever on the domed side marked CM. Place the adhesive side with a dimple against teeth or gums or braces or appliance. Place opposite or adjacent to the sore. Hold tablet in place with your cheek or tongue for 10 seconds to initiate adhesion. Adhesion will strengthen over the next few minutes. It may be removed at any time.
- Other Information
- Inactive Ingredients
- CankerMelts® 15mg carton of 20
-
INGREDIENTS AND APPEARANCE
CANKERMELTS
benzocaine lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68229-700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 15 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYLCELLULOSE (UNII: 9XZ8H6N6OH) HYALURONIC ACID (UNII: S270N0TRQY) PEPPERMINT OIL (UNII: AV092KU4JH) SODIUM BICARBONATE (UNII: 8MDF5V39QO) MAGNESIUM STEARATE (UNII: 70097M6I30) XYLITOL (UNII: VCQ006KQ1E) METHYLCOBALAMIN (UNII: BR1SN1JS2W) MALTODEXTRIN (UNII: 7CVR7L4A2D) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) ACACIA (UNII: 5C5403N26O) Product Characteristics Color white (bi-layered; slightly pink/off-white) Score no score Shape ROUND Size 12mm Flavor Imprint Code CM Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68229-700-20 2 in 1 CARTON 10/06/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 10/06/2023 Labeler - Quest Products, LLC (075402441) Establishment Name Address ID/FEI Business Operations Quest Products, LLC 116971363 manufacture(68229-700)
Trademark Results [CankerMelts]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CANKERMELTS 76356971 2856367 Live/Registered |
QUEST PRODUCTS, LLC 2002-01-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.