Bexagliflozin tablets. These highlights do not include all the information needed to use Bexagliflozin safely and effectively. See full prescribing information for Bexagliflozin. Bexagliflozin tablets, for oral use Initial U.S. Approval: 2023
Brenzavvy by
Drug Labeling and Warnings
Brenzavvy by is a Prescription medication manufactured, distributed, or labeled by Golden State Medical Supply, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BRENZAVVY- bexagliflozin tablet
Golden State Medical Supply, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONBexagliflozin tablets. These highlights do not include all the information needed to use Bexagliflozin safely and effectively. See full prescribing information for Bexagliflozin.
Bexagliflozin tablets, for oral use Initial U.S. Approval: 2023 INDICATIONS AND USAGEBexagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (
1)
DOSAGE AND ADMINISTRATION Recommended dose: 20 mg once daily, taken in the morning, with or without food. Do not crush or chew the tablet. (
2.2)
Withhold Bexagliflozin for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting ( 2.3). DOSAGE FORMS AND STRENGTHSTablets: 20 mg (3) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence > 5%) are female genital mycotic infections, urinary tract infection and increased urination ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact TheracosBio at 1-855-273-6928 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 9/2023 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Bexagliflozin is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use
Bexagliflozin is not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus.
[see Warnings and Precautions (
5.1)]
.
2 DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation and During Treatment with Bexagliflozin
Assess renal function prior to initiation of Bexagliflozin and periodically thereafter as clinically indicated [see Warnings and Precautions ( 5.3)] . Bexagliflozin is not recommended in patients with an eGFR less than 30 mL/min/1.73 m 2
Assess volume status. In patients with volume depletion, correct this condition before initiating Bexagliflozin [see Warnings and Precautions ( 5.3), Use in Specific Populations ( 8.5, 8.6)].
2.2 Recommended Dosage
The recommended dosage of Bexagliflozin is 20 mg orally taken once daily in the morning, with or without food
[see Clinical Pharmacology (
12.3)]
.
Do not crush or chew the tablet.
If a dose is missed, take the missed dose as soon as possible. Do not double the next dose.
2.3 Temporary Interruption for Surgery
Withhold Bexagliflozin for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting. Resume Bexagliflozin when the patient is clinically stable and has resumed oral intake [see Warnings and Precautions ( 5.1) and Clinical Pharmacology ( 12.2)] .
3 DOSAGE FORMS AND STRENGTHS
Tablets: 20 mg, blue, caplet-shaped, biconvex, bevel-edged, debossed with “2” and inverted “2” on one side.
4 CONTRAINDICATIONS
Bexagliflozin is contraindicated in patients:
- With hypersensitivity to bexagliflozin or any excipient in Bexagliflozin. Anaphylaxis and angioedema have been reported with sodium-glucose co-transporter 2 (SGLT2) inhibitors.
5 WARNINGS AND PRECAUTIONS
5.1 Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
In patients with type 1 diabetes mellitus, Bexagliflozin significantly increases the risk of diabetic ketoacidosis, a life-threatening event, beyond background rate. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was markedly increased in patients who received sodium glucose transporter 2 (SGLT2) inhibitors compared to patients who received placebo. Bexagliflozin is not indicated for glycemic control in patients with type 1 diabetes mellitus.
Type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are also risk factors for ketoacidosis. There have been postmarketing reports of fatal events of ketoacidosis in patients with type 2 diabetes mellitus using SGLT2 inhibitors.
Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include under-insulinization due to insulin dose reduction or missed insulin doses, acute febrile illness, reduced caloric intake, ketogenic diet, surgery, volume depletion, and alcohol abuse.
Signs and symptoms are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Blood glucose levels at presentation may be below those typically expected for diabetic ketoacidosis (e.g., less than 250 mg/dL). Ketoacidosis and glucosuria may persist longer than typically expected. Urinary glucose excretion persists for 3 days after discontinuing Bexagliflozin
[see Clinical Pharmacology (
12.2)]
; however, there have been postmarketing reports of ketoacidosis and/or glucosuria lasting greater than 6 days and some up to 2 weeks after discontinuation of SGLT2 inhibitors.
Consider ketone monitoring in patients at risk for ketoacidosis if indicated by the clinical situation. Assess for ketoacidosis regardless of presenting blood glucose levels in patients who present with signs and symptoms consistent with severe metabolic acidosis. If ketoacidosis is suspected, discontinue Bexagliflozin, promptly evaluate, and treat ketoacidosis, if confirmed. Monitor patients for resolution of ketoacidosis before restarting Bexagliflozin.
Withhold Bexagliflozin, if possible, in temporary clinical situations that could predispose patients to ketoacidosis. Resume Bexagliflozin when the patient is clinically stable and has resumed oral intake
[see Dosage and Administration (
2.3)]
.
Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue Bexagliflozin and seek medical attention immediately if signs and symptoms occur.
5.2 Lower Limb Amputation
An increased incidence of lower limb amputations occurred in Bexagliflozin-treated patients compared to placebo-treated patients (8.3 versus 5.1 events per 1,000 patient-years) in a randomized, placebo-controlled trial evaluating patients with type 2 diabetes who had either established cardiovascular disease (CVD) or were at risk for CVD (Trial 6). Additional amputation data from Trial 6 are shown in Table 2[see Adverse Reactions( 6.1)]. Of the 23 Bexagliflozin-treated patients who had amputations, 15 were amputations of the toe and midfoot and 8 were amputations above and below the knee. Some patients had multiple amputations.
Lower limb infections, gangrene, ischemia, and osteomyelitis were the most common precipitating medical events leading to the need for an amputation. The risk of amputation was highest in patients with a baseline history of prior amputation, peripheral vascular disease, and neuropathy.
Before initiating Bexagliflozin, consider factors in the patient history that may predispose to the need for amputations, such as a history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers. Counsel patients about the importance of routine preventative foot care. Monitor patients receiving Bexagliflozin for signs and symptoms of infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue Bexagliflozin if these complications occur.
5.3 Volume Depletion
Bexagliflozin can cause intravascular volume contraction which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions ( 6.1)] . There have been postmarketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m 2) [see Use in Specific Populations ( 8.6)] , elderly patients, patients with low systolic blood pressure, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating Bexagliflozin in patients with one or more of these characteristics, assess volume status and renal function [see Dosage and Administration ( 2.1)] . In patients with volume depletion, correct this condition before initiating Bexagliflozin. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy.
5.4 Urosepsis and Pyelonephritis
There have been reports of serious urinary tract infections, including urosepsis and pyelonephritis, requiring hospitalization in patients receiving SGLT2 inhibitors, including Bexagliflozin. Treatment with SGLT2 inhibitors, including Bexagliflozin, increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions ( 6.1)] .
5.5 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues (e.g., sulfonylureas) are known to cause hypoglycemia. Bexagliflozin may increase the risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue [see Adverse Reactions ( 6.1)] . A lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with Bexagliflozin.
5.6 Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier’s Gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with Bexagliflozin presenting with pain or tenderness, erythema, or swelling in the genital or perineal areas, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue Bexagliflozin, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.7 Genital Mycotic Infections
Bexagliflozin increases the risk of genital mycotic infections [see Adverse Reactions ( 6.1)] . Patients who have a history of genital mycotic infections or who are uncircumcised are more likely to develop genital mycotic infections. Monitor and treat appropriately.
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis [see Warnings and Precautions ( 5.1)]
- Lower Limb Amputation [see Warnings and Precautions ( 5.2) ]
- Volume Depletion [see Warnings and Precautions ( 5.3)]
- Urosepsis and Pyelonephritis [see Warnings and Precautions ( 5.4)]
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues [see Warnings and Precautions ( 5.5)]
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) [see Warnings and Precautions ( 5.6)]
- Genital Mycotic Infections [see Warnings and Precautions ( 5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials Evaluating Bexagliflozin 20 mg
The data in
Table 1 are derived from three trials in adults with type 2 diabetes mellitus: two 24-week placebo-controlled trials (one as monotherapy and another as add-on to metformin therapy; Trials 1 and 2, respectively)
[see Clinical Studies (
14.2,
14.3)]
and a 12-week, placebo-controlled, dose-ranging, monotherapy trial (only the data from the 20 mg dosage of Bexagliflozin per day were included in this pool). In these pooled trials, patients received placebo (N = 300) or Bexagliflozin 20 mg (N = 372), once daily. The mean age of the population was 56 years and 5% of the patients were older than 75 years of age. Fifty-seven percent (57%) were male and 45% were White, 38% Asian, 15% Black and 2% other races. At baseline, the mean duration of type 2 diabetes mellitus was 7.7 years and the mean hemoglobin A1c (HbA1c) was 8.2%. Established microvascular complications of type 2 diabetes mellitus at baseline included diabetic nephropathy (0.8%), retinopathy (24%), and peripheral neuropathy (33%). Baseline renal function was eGFR ≥ 60 mL/min/1.73 m
2in 98% of patients and eGFR 45 to < 60 mL/min/1.73 m
2in 2% of patients (mean eGFR 92 mL/min/1.73 m
2).
Table 1shows common adverse reactions associated with the use of Bexagliflozin in these trials. These adverse reactions occurred more commonly in Bexagliflozin-treated patients than placebo-treated patients, and occurred in at least 2% of Bexagliflozin-treated patients.
Table 1. Adverse Reactions in Adults with Type 2 Diabetes Mellitus - Monotherapy or in Combination with Metformin*
|
Percentage of Patients |
||
|
Placebo N = 300 |
Bexagliflozin N = 372 |
|
| Increased urination a |
3 |
7 |
| Urinary tract infection b |
4 |
6 |
| Female genital mycotic infections c |
0 |
6 |
| Thirst d |
2 |
3 |
| Vaginal pruritus e |
0 |
3 |
| Male genital mycotic infection f |
1 |
2 |
| Hypoglycemia |
1 |
2 |
*The three placebo-controlled trials included two monotherapy trials and one add-on combination trial with metformin in adults with type 2 diabetes mellitus (Trials 1, 2, and a 12-week dose ranging trial). Adverse reactions were those that occurred more commonly in Bexagliflozin-treated patients than placebo-treated patients and occurred in at least 2% of Bexagliflozin-treated patients.
aIncludes: polyuria, pollakiuria, micturition urgency, nocturia.
bIncludes: dysuria, urinary tract infection, nitrite urine present, streptococcal urinary tract infection, cystitis.
cIncludes: vulvovaginal mycotic infection, vaginal infection, genital infection fungal, vulvovaginal candidiasis. Percentages calculated with the number of female patients in each group as denominator: placebo (N = 130), Bexagliflozin (N = 156).
dIncludes: thirst, polydipsia.
eIncludes: pruritus genital, vulvovaginal pruritus. Percentages calculated with the number of females in each group as denominator: placebo (N = 130), Bexagliflozin (N = 156).
fIncludes: balanoposthitis, genital infection fungal, tinea cruris. Percentages calculated with the number of males in each group as denominator: placebo (N = 170), Bexagliflozin (N = 216).
Clinical Trial in Patients with Increased Risk for Major Adverse Cardiovascular Events
Bexagliflozin was evaluated in a trial that enrolled adults with type 2 diabetes mellitus who had either established (CVD) or were at increased risk for CVD (Trial 6)
[see Clinical Studies (
14.5)]
. Patients on standard of care therapy for diabetes management were randomized to receive add-on therapy with either placebo (N = 567) or Bexagliflozin 20 mg once daily (N = 1,132) for a minimum duration of 52 weeks (median duration 2.4 years). The most common adverse reactions observed in this trial were generally consistent with other trials of Bexagliflozin in adults with type 2 diabetes mellitus
(see
Table 1)
.
Other Adverse Reactions
Lower Limb Amputations
An increased incidence of non-traumatic lower limb amputations occurred in Bexagliflozin-treated patients compared to placebo-treated patients in a trial (Trial 6) that evaluated adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD. Patients in this trial were followed for a median duration of 2.4 years. The lower limb amputation data are shown in
Table 2.
Table 2. Non-traumatic Lower Limb Amputation in Adults with Type 2 Diabetes Mellitus who had either Established Cardiovascular Disease or were at Risk for Cardiovascular Disease (Trial 6)
|
Placebo N = 567 |
Bexagliflozin N = 1,132 |
|
| Patients with an amputation, n (%) |
7 (1.2%) |
23 (2.0%) |
| Total amputations |
13 |
25 |
| Amputation incidence rate (per 1,000 patient-years) |
5.1 |
8.3 |
| Hazard Ratio (95% CI) |
- |
1.64 (0.70, 3.82) |
Note: Incidence is based on the number of patients with at least one amputation, and not the total number of amputation events. A patient’s follow-up is calculated from Day 1 to the first amputation event date. Some patients had more than one amputation.
Volume Depletion
In a trial of adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), adverse reactions related to volume depletion (e.g., dehydration, dizziness, dizziness postural, vertigo, vertigo positional, presyncope, hypotension, and orthostatic hypotension) were reported in 3.9% and 8.9% of patients treated with placebo and Bexagliflozin, respectively.
Genital Mycotic Infections
In a pool of three placebo-controlled clinical trials (12-week dose ranging trial and Trials 1 and 2), the incidence of female genital mycotic infections occurred in 0% and 5.6% of females treated with placebo and Bexagliflozin, respectively (see
Table 1). In the same pool of trials, male genital mycotic infections occurred in 1.4% and 2.2% of males treated with placebo and Bexagliflozin, respectively (see
Table 1). In a trial that enrolled adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), 0% and 9.2% of female patients treated with placebo and Bexagliflozin, respectively, had a genital mycotic infection.
In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), 2.8% and 9.0% of patients treated with placebo and Bexagliflozin, respectively, had at least one event of genital mycotic infection. In the same trial, genital mycotic infections that caused drug discontinuation were reported in 0% and 1.2% of patients treated with placebo and Bexagliflozin, respectively. Balanoposthitis was reported in 0% and 2.9% of male patients, and phimosis was reported in 0.3% and 0.5% of male patients treated with placebo and Bexagliflozin, respectively. Patients treated with Bexagliflozin with events of phimosis typically underwent circumcision.
Fractures
In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), the incidence rates of serious fractures, including events of hip and femur fracture, were 1.4 and 5.4 events per 1,000 patient-years of follow-up in the placebo and Bexagliflozin groups, respectively. The imbalance in serious fractures was observed within the first 6 months of therapy and remained through the end of the trial.
Hypoglycemia
The incidence of hypoglycemia by trial is shown in
Table 3.
Table 3. Incidence of Overall *and Severe †Hypoglycemia in Placebo-Controlled Clinical Trials in Adults with Type 2 Diabetes Mellitus
|
Placebo |
Bexagliflozin |
|
| Monotherapy (24 weeks) (Trial 1) | ||
|
All subjects -Overall [N (%)] -Severe [N (%)] |
N = 69 0 (0) 0 (0) |
N = 138 0 (0) 0 (0) |
| Add-on to Metformin (24 weeks) (Trial 2) | ||
| All subjects
-Overall [N (%)] -Severe [N (%)] |
N = 159 0 (0) 0 (0) |
N = 158 1 (0.6) 0 (0) |
| Add-on to Standard of Care Therapy in Patients with Moderate Renal Impairment (24 weeks) (Trial 5) β | ||
| All subjects
-Overall [N (%)] -Severe [N (%)] Subjects on background insulin and/or sulfonylurea -Overall [N (%)] -Severe [N (%)] |
N = 155 0 (0) 0 (0) N = 109 0 (0) 0 (0) |
N = 157 2 (1.3) 1 (0.6) N = 106 2 (1.9) 1 (0.9) |
| Add-on to Standard of Care Therapy in Patients with Increased CV Risk (Trial 6) β | ||
| All subjects
-Overall [N (%)] -Severe [N (%)] Subjects on background insulin and/or sulfonylurea -Overall [N (%)] -Severe [N (%)] |
N = 567 11 (1.9) 8 (1.4) N = 454 10 (2.2) 8 (1.8) |
N = 1,132 23 (2.0) 10 (0.9) N = 923 22 (2.4) 10 (1.1) |
*Overall hypoglycemia: plasma or capillary glucose of less than 54 mg/dL.
†Severe hypoglycemia: patient required assistance, lost consciousness, or experienced a seizure (irrespective of blood glucose concentration).
βNo restrictions were placed on background antihyperglycemic therapy (aside from treatment with another SGLT2 inhibitor) and approximately 50% of patients used insulin and/or an insulin secretagogue at baseline.
Rash and Dermatitis
In the clinical program of Bexagliflozin, one event of rash and one event of dermatitis was confirmed to be attributable to Bexagliflozin exposure by withdrawal and rechallenge. The rash and dermatitis events occurred on day 37 and day 3 of exposure to Bexagliflozin, respectively. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), 3.4% and 5.4% of patients experienced at least one event of rash with placebo and Bexagliflozin, respectively.
Sepsis
Bexagliflozin was associated with an increased risk of sepsis/septic shock events, including events that may have caused or contributed to death, in a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6). Sepsis events occurred in 2 (0.4%) and 14 (1.2%) of placebo-treated patients and Bexagliflozin-treated patients in the trial, respectively. Of these, 1 sepsis event among placebo-treated patients and 3 sepsis events among Bexagliflozin-treated patients were related to urinary tract infections.
Laboratory Abnormalities
Changes in Serum Creatinine and eGFR
Initiation of Bexagliflozin causes an increase in serum creatinine and decrease in eGFR within weeks of starting therapy that stabilizes by week 6 to 12. In a trial enrolling adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), a mean change in serum creatinine of 0.0 mg/dL and a decrease in eGFR of 0.1 mL/min/1.73 m
2 was observed in the placebo group as compared to a mean increase in serum creatinine of 0.1 mg/dL and a mean decrease in eGFR of 4.6 mL/min/1.73 m
2with Bexagliflozin, within the first 6 weeks of treatment. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), an initial decrease in eGFR was seen within weeks of starting therapy (eGFR changes from baseline to week 12 of 0 and -3.1 mL/min/1.73 m
2in the placebo and Bexagliflozin arms, respectively). Acute hemodynamic changes may play a role in the early renal function changes observed with Bexagliflozin since they are reversed after treatment discontinuation.
Increases in Low-Density Lipoprotein Cholesterol (LDL-C)
In a pool of two placebo-controlled clinical trials (Trials 1 and 2), mean LDL-C decreased by 3.8 mg/dL (3.7%) in patients treated with placebo (N = 195) and increased by 1.7 mg/dL (1.6%) in patients treated with Bexagliflozin (N = 247) at week 24. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), LDL-C increased by 3 mg/dL (3.2%) and 3 mg/dL (4.1%) with placebo and Bexagliflozin treatment, respectively, at Week 24.
Increases in Hemoglobin and Hematocrit
In a pool of two placebo-controlled trials (Trials 1 and 2), mean changes from baseline to Week 24 in hemoglobin were -0.3 g/dL (-2.1%) with placebo and 0.4 g/dL (2.9%) with Bexagliflozin. In the same pool, mean changes from baseline to Week 24 in hematocrit were -0.6% with placebo and 1.3% with Bexagliflozin 20 mg. Fewer patients had > 2 g/dL increases in hemoglobin from baseline for placebo (0.5%) compared to Bexagliflozin (4.9%). Increases in hemoglobin > 3 g/dL from baseline were observed in 0% of placebo-treated patients compared to 0.7% of Bexagliflozin-treated patients.
7 DRUG INTERACTIONS
See Table 4 for clinically significant interactions with Bexagliflozin.
Table 4. Clinically Significant Interactions with Bexagliflozin
| UGT Enzyme Inducers | |
| Clinical Impact | UGT Enzyme Inducers may significantly reduce exposure to Bexagliflozin and lead to a decreased efficacy [ see Clinical Pharmacology ( 12.3)]. |
| Intervention | Consider adding another antihyperglycemic agent in patients who require additional glycemic control. |
| Concomitant Use with Insulin and Insulin Secretagogues | |
| Clinical Impact | The risk of hypoglycemia is increased when Bexagliflozin is used in combination with insulin and/or an insulin secretagogue. |
| Intervention | A lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with Bexagliflozin. |
| Lithium | |
| Clinical Impact | Concomitant use with SGLT2 inhibitors such as Bexagliflozin may decrease serum lithium concentrations. |
| Intervention | Monitor serum lithium concentration more frequently upon Bexagliflozin initiation and discontinuation. |
| Positive Urine Glucose Test | |
| Clinical Impact | SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. |
| Intervention | Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
| Interference with 1,5-anhydroglucitol (1,5-AG) Assay | |
| Clinical Impact | Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
| Intervention | Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data showing adverse renal effects, Bexagliflozin is not recommended during the second and third trimesters of pregnancy.
The available data on use of Bexagliflozin during pregnancy are insufficient to determine a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations) .
In animal studies, adverse renal pelvic and tubule dilatations that were not fully reversible were observed in rats when bexagliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy at exposures 11 times the 20 mg clinical dose
(see
Data)
.
The estimated background risk of major birth defects is 6% to 10% in women with pre-gestational diabetes with a peri-conceptional HbA1c > 7% and has been reported to be as high as 20% to 25% in women with a peri-conceptional HbA1c > 10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Hypoglycemia and hyperglycemia occur more frequently during pregnancy in patients with pre-gestational diabetes. Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Animal Data
Bexagliflozin administered to juvenile rats at 0.3, 3 or 30 mg/kg/day by oral gavage from postnatal days 21 to 90 caused a dose dependent increase in the incidence and severity of renal pelvic and tubular dilatation at ≥ 3 mg/kg (11 times the clinical dose of 20 mg based on AUC). These outcomes occurred with drug exposure during periods of renal development in rats equivalent to the late second and third trimester of human renal development and did not fully reverse following a 1-month recovery period.
In embryofetal development studies in rats and rabbits, bexagliflozin was administered at 7, 40, and 200 mg/kg/day (rats) and 5, 25, and 150 mg/kg/day (rabbits) during organogenesis. No adverse developmental effects were observed in rats at doses up to 200 mg/kg/day (551 times the clinical dose of 20 mg based on AUC). Reduced maternal body weight, embryo lethality, and fetal malformations were observed in rabbits at 150 mg/kg/day (368 times the clinical dose of 20 mg based on AUC).
In a prenatal and postnatal development study, bexagliflozin was administered to maternal rats by oral gavage during organogenesis and until weaning at doses of 7, 40, or 200 mg/kg/day. Maternal mortality occurred at ≥ 40 mg/kg (79 times the clinical dose of 20 mg based on AUC), primarily following parturition. Reduced gestational body weight, increased post-implantation loss, and smaller litter size were noted at 200 mg/kg (361 times the clinical dose of 20 mg based on AUC). In the offspring, lower body weight gain and decreased survival were noted at 200 mg/kg, which occurred in the presence of significant maternal toxicity.
8.2 Lactation
Risk Summary
There is no information regarding the presence of bexagliflozin in human milk, the effects on the breastfed infant or the effects on milk production. Bexagliflozin is excreted in the milk of lactating rats
(see
Data)
. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney. Because of the potential for serious adverse reactions in a breastfed infant, including the potential for bexagliflozin to affect postnatal renal development, advise patients that use of Bexagliflozin is not recommended while breastfeeding.
Data
Bexagliflozin was present in rat milk at a milk:plasma ratio of approximately 2. The concentration of bexagliflozin in animal milk does not necessarily predict the concentration of bexagliflozin in human milk.
Juvenile rats directly exposed to bexagliflozin showed a risk to the developing kidney (renal pelvic and tubular dilatation) during the period of renal development in rats corresponding to the late second and third trimester of human renal development.
8.4 Pediatric Use
The safety and effectiveness of Bexagliflozin have not been established in pediatric patients.
8.5 Geriatric Use
In 9 clinical trials of Bexagliflozin, 1047 (40.6%) patients 65 years and older, and 212 (8.2%) patients 75 years and older were exposed to Bexagliflozin [see Clinical Studies ( 14)] .
One of the 9 trials enrolled patients with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), and had a total of 571 (50%) patients treated with Bexagliflozin who were 65 years and older, and 113 (10%) patients treated with Bexagliflozin who were 75 years and older [see Clinical Studies ( 14.5)] . No overall differences in the effectiveness of Bexagliflozin have been observed between patients 65 years of age and older and younger adult patients. Among patients aged 65 and older in this trial, volume depletion events were reported in 7.6% and 9.8% of patients in the placebo and Bexagliflozin groups, respectively [see Warnings and Precautions ( 5.3)] .
8.6 Renal Impairment
Bexagliflozin is not recommended in patients with an eGFR less than 30 mL/min/1.73 m 2 due to the decline of the glucose lowering effect of Bexagliflozin and reduction in urine output in these patients [see Clinical Pharmacology ( 12.3)] . The recommended dosage for patients with an eGFR greater than or equal to 30 mL/min/1.73 m 2 is the same as the recommended dosage for patients with normal renal function [see Dosage and Administration ( 2.1)] .
The safety and efficacy of Bexagliflozin in adults with type 2 diabetes mellitus and moderate renal impairment (eGFR between 30 and 60 mL/min/1.73 m 2) were evaluated in Trial 5 [see Clinical Studies ( 14.4)] . Efficacy and safety studies with Bexagliflozin did not enroll patients with an eGFR less than 30 mL/min/1.73 m 2.
Bexagliflozin-treated patients with renal impairment may be more likely to experience adverse reactions associated with Bexagliflozin treatment, including female genital mycotic infection, increased urination, and thirst, and may be at higher risk for volume depletion and acute kidney injury [see Warnings and Precautions ( 5.3) and Adverse Reactions ( 6.1)] .
8.7 Hepatic Impairment
Bexagliflozin has not been studied in patients with severe hepatic impairment and is not recommended for use in this patient population. The recommended dosage for patients with mild to moderate hepatic impairment is the same as the recommended dosage for patients with normal hepatic function [see Clinical Pharmacology ( 12.3)] .
10 OVERDOSAGE
In the event of an overdose of Bexagliflozin, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. Employ the usual supportive measures as dictated by the patient’s clinical status. Removal of bexagliflozin by hemodialysis has not been studied.
11 DESCRIPTION
Bexagliflozin tablets for oral use contain bexagliflozin, an SGLT2 inhibitor.
The chemical name of bexagliflozin is (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.
The molecular formula is C
24H
29ClO
7and the molecular weight is 464.94 g/mol. The structural formula is:
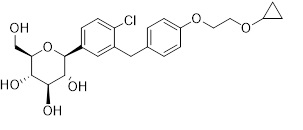
Bexagliflozin is a white/off-white to pale yellow powder. It is very slightly soluble in water and freely soluble in methanol, acetone, ethylene glycol, and propylene glycol. It is slightly soluble in heptane, cyclohexane, and toluene. Crystalline bexagliflozin is not hygroscopic.
Each film-coated tablet contains 20 mg of bexagliflozin and the inactive ingredients colloidal silicon dioxide, glyceryl dibehenate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene oxide, and poloxamer 188. In addition, the film coating ingredient, Opadry
®II Blue 85F99153, contains the inactive ingredients FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, macrogol 3350, partially hydrolyzed polyvinyl alcohol, talc, and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bexagliflozin is an inhibitor of sodium-glucose co-transporter 2 (SGLT2), the transporter responsible for reabsorption of the majority of glucose from the renal glomerular filtrate in the renal proximal tubule. By inhibiting SGLT2, bexagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
12.2 Pharmacodynamics
Urinary Glucose Excretion and Urinary Volume
Dose-dependent increases in urinary glucose excretion (UGE) accompanied by increases in urine volume were observed in healthy subjects and in adults with type 2 diabetes mellitus following single- and multiple-dose administration of bexagliflozin. Dose-response analysis indicates that 20 mg bexagliflozin provides near-maximal UGE. Elevated UGE was maintained after multiple-dose administration.
Cardiac Electrophysiology
At 5 times the recommended dose, bexagliflozin does not prolong the QTc interval to any clinically significant extent.
12.3 Pharmacokinetics
The pharmacokinetics of bexagliflozin are similar in healthy subjects and adults with type 2 diabetes mellitus. Following dosing in the fasted state, mean C maxand AUC 0-∞were 134 ng/mL and 1,162 ng·h/mL, respectively. Bexagliflozin does not exhibit time-dependent pharmacokinetics and accumulates in plasma up to ~20% following multiple dosing.
Absorption
Following oral administration of Bexagliflozin, peak plasma concentrations of bexagliflozin were reached between 2 – 4 hours post-dose and can be delayed if taken after a meal or by medications that slow gastric emptying. Plasma C
maxand AUC of bexagliflozin increase in a dose-proportional manner following single doses from 3 mg (0.15 times the recommended dose) to 90 mg (4.5 times the recommended dose).
Effect of Food
Administration of Bexagliflozin after consumption of a standard high-fat, high-caloric meal increased C
maxand AUC by 31% and 10%, respectively, compared to dosing in the fasted state. The median T
maxwas increased to 5 hours. The effects of food on bexagliflozin pharmacokinetics are not considered clinically relevant
[see Dosage and Administration (
2.2)]
.
Distribution
Bexagliflozin is approximately 93% bound to plasma protein. Neither renal nor hepatic impairment substantially alters protein binding. The apparent volume of distribution is 262 L.
Elimination
Metabolism
Bexagliflozin is mainly metabolized by UGT1A9 and, to a lesser extent, CYP3A. In plasma the most abundant metabolite is the pharmacologically inactive 3′-
O-glucuronide, which was found to constitute 32.2% of the parent compound AUC in a radiolabeled tracer study. None of the metabolites are expected to have clinically relevant pharmacological effects.
Excretion
The apparent oral clearance of bexagliflozin is 19.1 L/h by population pharmacokinetic modeling. The apparent terminal elimination half-life of bexagliflozin was approximately 12 hours. Following administration of an oral [
14C]-bexagliflozin solution to healthy subjects, 91.6% of input radioactivity was recovered, 51.1% in feces, the majority as bexagliflozin, and 40.5% in urine, largely as the 3′-
O-glucuronide. The proportion of input radioactivity recovered as bexagliflozin in urine and feces was 1.5% and 28.7%, respectively
Specific Populations
Patients with Renal Impairment
In a clinical pharmacology study in patients with type 2 diabetes mellitus and mild (eGFR 60 to 89 mL/min/1.73 m
2), moderate (eGFR 30 to 59 mL/min/1.73 m
2), and severe (eGFR less than 30 mL/min/1.73 m
2) renal impairment, the AUC of bexagliflozin was 7%, 34% and 54% greater than in patients with normal renal function, respectively, after administration of a single 20 mg dose of Bexagliflozin. These increases in bexagliflozin AUC are not considered clinically meaningful.
Consistent with the mechanism of action of bexagliflozin, the 24-hour UGE in patients with type 2 diabetes mellitus and mild, moderate, and severe renal impairment was 17%, 60%, and 83% lower than in patients with type 2 diabetes mellitus with normal renal function, respectively. Therefore, the glucose-lowering pharmacodynamic response to bexagliflozin declines with increasing severity of renal impairment
[see Dosage and Administration (
2.1), Warnings and Precautions (
5.3), Use in Specific Populations (
8.6), and Clinical Studies (
14)]
. The impact of hemodialysis on bexagliflozin exposure is not known.
Patients with Hepatic Impairment
In patients with moderate hepatic impairment (Child-Pugh class B), the AUC of bexagliflozin increased by 28%, and C
maxincreased by 6.3% compared to subjects with normal hepatic function. These increases in bexagliflozin AUC and C
maxare not considered clinically meaningful. There is no clinical experience in patients with Child-Pugh class C (severe) hepatic impairment
[see Use in Specific Populations (
8.7)
Effects of Age, Body Weight, Sex, and Race
Based on a population pharmacokinetic analysis, age, body weight, sex and race do not have a clinically relevant effect on the pharmacokinetics of bexagliflozin.
Drug Interaction Studies
In vitro Assessment of Drug Interactions
Based on
in vitrostudies, bexagliflozin is not expected to inhibit CYP450 isoenzymes (CYPs) 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4, or induce CYPs 1A2, 2C19 and 3A4 at clinically relevant plasma concentrations.
Bexagliflozin is not expected to inhibit drug transporters including breast cancer resistance protein (BRCP), bile salt export pump (BSEP), organic anion transporting polypeptides (OATP1B1, OATP1B3), anion transporters (OAT1, OAT3), organic cation transporters (OCT1, OCT2), and multidrug and toxin extrusion transporters (MATE1, MATE2-K) at clinically relevant plasma concentrations. Bexagliflozin is a substrate for P-glycoprotein (P-gp)
.
In vivo Assessment of Drug Interactions
There are no clinically meaningful changes in bexagliflozin exposure when taken with metformin, glimepiride, sitagliptin, exenatide, probenecid, or verapamil (
Figure 1). Bexagliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, sitagliptin, and digoxin (
Figure 2).
Figure 1. Effect of Other Drugs on the Pharmacokinetics of Bexagliflozin
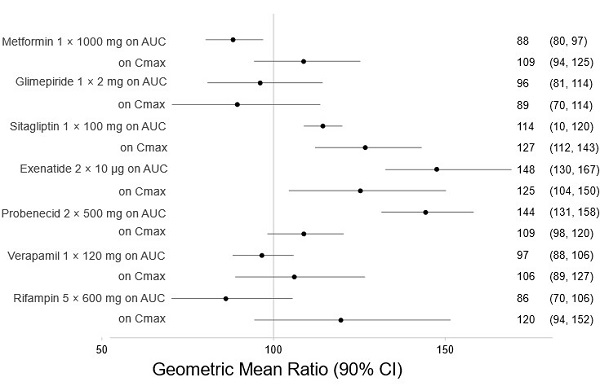
Note: Rifampin dosing over 5 days, the regimen may not represent the maximal impact on bexagliflozin exposure. All others were single day dosing (once daily or twice daily).
Figure 2. Effect of Bexagliflozin on the Pharmacokinetics of Other Drugs
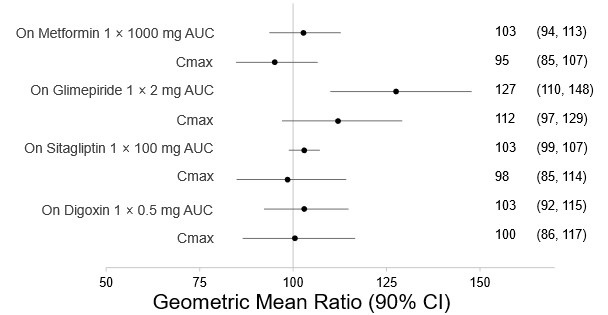
Note: Single day dosing.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenesis was evaluated in 2-year studies in CD-1 mice at oral gavage doses of 15, 50, and 150 mg/kg/day and in Sprague-Dawley (SD) rats at 3, 10, and 30 mg/kg/day. In male rats, the dose was reduced to 1, 3, and 10 mg/kg/day at week 73 due to exacerbation of chronic progressive nephropathy resulting in excessive mortality. There were no drug-related neoplastic findings in mice or rats at up to the highest doses tested representing up to 156 times (mice) and 68 times (rats) the clinical dose of 20 mg based on AUC.
Mutagenesis
Bexagliflozin was not mutagenic or clastogenic with or without metabolic activation in the
in vitroAmes bacterial mutagenicity assay, the
in vitroCHO cell assay, an
in vivomicronucleus assay in rats, and an
in vivounscheduled hepatic DNA synthesis study in rats.
Impairment of Fertility
Bexagliflozin had no effects on mating, fertility or early embryonic development in male or female rats at any dose up to the highest dose of 200 mg/kg/day, which resulted in exposures 280 times (males) and 439 times (females) the 20 mg clinical dose (based on AUC).
14 CLINICAL STUDIES
14.1 Overview of Clinical Trials
Bexagliflozin has been studied as monotherapy (Trial 1) and in combination with metformin in adults with type 2 diabetes mellitus (Trials 2, 3, and 4)
[see Clinical Studies (
14.2and
14.3)]
. Bexagliflozin has also been studied in adults with type 2 diabetes mellitus with moderate renal impairment (Trial 5)
[see Clinical Studies (
14.4)]
, and in adults with type 2 diabetes mellitus with established CVD or at increased risk for CVD (Trial 6)
[see Clinical Studies (
14.5)]
.
Treatment with Bexagliflozin reduced hemoglobin A1c (HbA1c) compared to placebo and efficacy was noninferior to glimepiride (up-titrated to a maximum dose of 6 mg) and sitagliptin 100 mg once daily (see Trials 3 and 4). The reduction in HbA1c by Bexagliflozin was shown across subgroups of age, sex, race, and geographic region.
14.2 Glycemic Control in Adults with Type 2 Diabetes Mellitus - Monotherapy (Trial 1)
A total of 207 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) by diet and exercise participated in a randomized, double-blind, multi-center, 24-week, placebo-controlled trial (NCT02715258; referred to as Trial 1) to evaluate the efficacy of Bexagliflozin monotherapy. Patients were either treatment naïve or had discontinued a single oral antihyperglycemic treatment ≥ 6 weeks prior to entering a 2-week, single-blind, placebo run-in period. Upon completion of the run-in period they were randomized (1:2) to placebo or Bexagliflozin 20 mg administered orally once daily. The mean age of the population was 55 years and 4% of the patients were older than 75 years of age. Forty-eight percent (48%) were male and 74% were White, 10% were Asian, 15% were Black and 1% were other races. Fifty-two percent (52%) were Hispanic/Latino.
At week 24, treatment with Bexagliflozin provided a statistically significant reduction in HbA1c compared to placebo (see Table 5).
Table 5. Glycemic Results from a 24-Week Placebo-Controlled Monotherapy Trial of Bexagliflozin in Adults with Type 2 Diabetes Mellitus (Trial 1)
|
Placebo N = 69 |
Bexagliflozin N = 138 |
|
| HbA1c (%) | ||
| Baseline mean |
7.9 |
8.1 |
| Change from baseline [adjusted mean (SE)] a |
-0.1 (0.1) |
-0.5 (0.1) |
| Difference from placebo [adjusted mean] (95% CI) |
-0.4 (-0.6, -0.1) * |
|
| Proportion of patients (%) achieving HbA1c <7% b |
20% |
31% |
| FPG (mg/dL) | ||
| Baseline mean |
170 |
169 |
| Change from baseline [adjusted mean (SE)] c |
-3 (4) |
-16 (3) |
| Difference from placebo [adjusted mean] (95% CI) |
-14 (-24, -3) |
|
SE: Standard Error; CI: Confidence Interval; FPG: Fasting plasma glucose
*Statistically significant (multiplicity adjusted one-sided p-value < 0.025)
aIntention to treat population. ANCOVA was used to analyze data using imputed values by return to baseline analysis for missing data at week 24 (9% and 7% for bexagliflozin and placebo, respectively). The ANCOVA model included treatment, country, background anti-diabetes treatment status (treatment naïve or not) and the baseline HbA1c value as a covariate.
bCrude proportion using imputed HbA1c values for missing data at week 24 and averaged across multiply imputed datasets
cSame model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
Figure 3. Mean Change from Baseline in HbA1c (%) by Time and Treatment
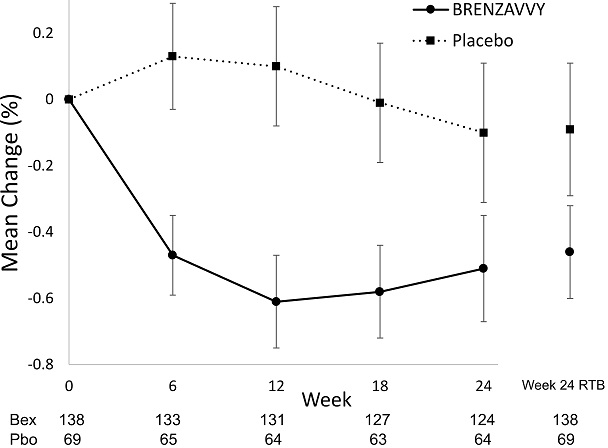
Vertical lines represent 95% confidence intervals. Treatment group least squares mean changes from baseline in HbA1c were estimated by an ANCOVA model using observed data for intermediate visits. Numbers of patients per arm per measurement shown below plot. Return-to-baseline (RTB) analysis results at Week 24 are plotted to the right separately.
14.3 Glycemic Control in Adults with Type 2 Diabetes Mellitus – Combination Therapy (Trials 2, 3 and 4)
Add-on Combination Therapy with Metformin (Trial 2)
A total of 317 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7.5% and 10.5%) by metformin monotherapy (≥ 1,000 mg/day or ≥ 1,500 mg/day for ≥ 8 weeks depending on country) participated in a randomized, double-blind, multi-center, 24-week, placebo-controlled trial (NCT03259789; referred to as Trial 2) to evaluate the efficacy of Bexagliflozin in combination with metformin. Patients entered a 1-week, single-blind, placebo run-in period, and were randomized (1:1) to placebo or Bexagliflozin 20 mg administered orally once daily in addition to the background metformin therapy. The mean age of the population was 56 years and 4% of the patients were older than 75 years of age. Sixty-one percent (61%) were male and 31% were White, 50% were Asian, 17% were Black and 2% were other races. Twenty-one percent (21%) were Hispanic/Latino.
At Week 24, treatment with Bexagliflozin provided a statistically significant reduction in HbA1c compared to placebo (see
Table 6).
Table 6. Glycemic Results From a 24-Week Placebo-Controlled Trial for Bexagliflozin used in Combination with Metformin in Adults with Type 2 Diabetes Mellitus (Trial 2)
|
Placebo N = 159 |
Bexagliflozin N = 158 |
|
| HbA1c (%) | ||
| Baseline mean |
8.5 |
8.6 |
| Change from baseline [adjusted mean (SE)] a |
-0.5 (0.1) |
-1.0 (0.1) |
| Difference from placebo [adjusted mean] (95% CI) |
-0.5 (-0.7, -0.3) * |
|
| Proportion of patients (%) achieving HbA1c < 7% b |
10% |
26% |
|
FPG (mg/dL) |
||
| Baseline mean |
190 |
186 |
| Change from baseline [adjusted mean (SE)] c |
-20 (3) |
-42 (3) |
| Difference from placebo [adjusted mean] (95% CI) |
-22 (-31, -12) |
|
SE: Standard Error; CI: Confidence Interval; FPG: Fasting plasma glucose
*Statistically significant (multiplicity adjusted one-sided p-value < 0.025)
aIntention to treat population. ANCOVA was used to analyze data using imputed values by return to baseline analysis for missing data at week 24 (10% and 9% for bexagliflozin and placebo, respectively). The ANCOVA model included treatment, baseline HbA1c value and country (US or Japan).
bCrude proportion using imputed HbA1c values for missing data at week 24 and averaged across multiply imputed datasets
cSame model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
Active-Controlled Trial versus Glimepiride as Add-on Therapy with Metformin (Trial 3)
A total of 426 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) by metformin monotherapy participated in a randomized, double-blind, multi-center, 60-week, active comparator-controlled trial (NCT02769481; referred to as Trial 3) to evaluate the efficacy of Bexagliflozin in combination with metformin. Patients receiving metformin (≥ 1,500 mg/day) as monotherapy entered a 2-week, single-blind, placebo run-in period; patients receiving metformin and another oral hypoglycemic agent enrolled if they discontinued the second agent for at least 6 weeks. Upon completion of the run-in period, they were randomized (1:1) to glimepiride or Bexagliflozin 20 mg administered orally once daily in addition to the background metformin therapy. Glimepiride therapy was initiated at 2 mg/day and titrated up to 6 mg/day or the maximum tolerated dose below 6 mg/day. Glimepiride up-titration occurred until week 6 of the 60-week treatment period. The mean daily dose of glimepiride was 5.4 mg (the maximal approved dosage in the United States is 8 mg per day). Eighty-one percent of patients in the glimepiride group were titrated up to 6 mg per day. The mean age of the population was 60 years and 5% of the patients were older than 75 years of age. Fifty-eight percent (58%) were male and 94% were White, 3% were Asian, 2% were Black, and 1% were other races. Twenty-two percent (22%) were Hispanic/Latino.
Glycemic Results
Bexagliflozin was non-inferior to glimepiride in the change in HbA1c from baseline after 60 weeks of treatment (See
Table 7).
Table 7. Glycemic Results from a 60-Week Active-Controlled Trial Comparing Bexagliflozin to Glimepiride as an Add-On Therapy in Adults with Type 2 Diabetes Mellitus Inadequately Controlled by Metformin (Trial 3)
|
Glimepiride N = 213 |
Bexagliflozin N = 213 |
|
| HbA1c (%) | ||
| Baseline mean |
8.0 |
8.0 |
| Change from baseline [adjusted mean (SE)] a |
-0.6 (0.1) |
-0.7 (0.1) |
| Difference from glimepiride [adjusted mean] (95% CI) |
-0.0 (-0.2, 0.1) b |
|
| Proportion of patients (%) achieving HbA1c < 7% c |
33% |
35% |
| FPG (mg/dL) | ||
| Baseline mean |
174 |
172 |
|
Change from baseline [adjusted mean (SE)] d |
-14 (3) |
-22 (2) |
| Difference from glimepiride [adjusted mean] (95% CI) |
-8 (-15, -1) |
|
SE: Standard Error; CI: Confidence Interval; FPG: Fasting plasma glucose
aIntention to treat population. ANCOVA was used to analyze data using imputed values by return to baseline analysis for missing data at week 60 (9% and 10% for bexagliflozin and glimepiride, respectively). The ANCOVA model included treatment, region, background treatment status (metformin-only or metformin + another oral hypoglycemic agent), eGFR at baseline (≥ 90 vs. < 90 mL/min/1.73 m
2), and baseline HbA1c value. Non-inferiority is declared if the upper bound of the 95% confidence interval for the difference from glimepiride lies below 0.35.
bNon-inferior
cCrude proportion using imputed HbA1c values for missing data at week 60 and averaged across multiply imputed datasets
dSame model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
Body Weight and Systolic Blood Pressure Results
The mean baseline body weight in patients with baseline BMI ≥ 25 kg/m
2was 92 kg (N = 202) and 90 kg (N = 201) in the glimepiride and Bexagliflozin groups, respectively. The mean changes from baseline to Week 60 in this population were 0.6 kg and -3.4 kg in the glimepiride and Bexagliflozin groups, respectively. The difference from glimepiride (95% CI) for Bexagliflozin was -4.0 kg (-4.8, -3.2).
The mean baseline cuff systolic blood pressure (SBP) in patients with baseline values ≥ 140 mmHg was 149 mm Hg in the glimepiride (N = 75) and Bexagliflozin (N = 78) groups. The mean changes in cuff SBP in this population from baseline to Week 60 were -6.2 mmHg and -12.9 mmHg in the glimepiride and Bexagliflozin groups, respectively. The difference from glimepiride (95% CI) for Bexagliflozin was -6.8 mmHg (-10.8, -2.6).
Active-Controlled Trial versus Sitagliptin as Add-on Therapy with Metformin (Trial 4)
A total of 384 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 11%) by metformin monotherapy participated in a randomized, double-blind, multi-center, 24-week, active comparator-controlled trial (NCT03115112; referred to as Trial 4) to evaluate the efficacy of Bexagliflozin in combination with metformin. Patients receiving metformin monotherapy (≥ 1,500 mg/day for ≥ 8 weeks) entered a 1-week, single-blind, placebo run-in period and were randomized to sitagliptin 100 mg or Bexagliflozin 20 mg administered orally once daily in addition to the background metformin therapy. The mean age of the population was 59 years and 4% of the patients were older than 75 years of age. Sixty-four percent (64%) were male and 82% were White, 16% were Asian, and 2% were Black. Three percent (3%) were Hispanic/Latino.
Bexagliflozin was non-inferior to sitagliptin in the change in HbA1c from baseline after 24 weeks of treatment.
Table 8. Glycemic Results from a 24-Week Active-Controlled Trial Comparing Bexagliflozin to Sitagliptin as an Add-On Therapy in Adults with Type 2 Diabetes Mellitus Inadequately Controlled by Metformin (Trial 4)
|
Sitagliptin N = 193 |
Bexagliflozin N = 191 |
|
| HbA1c (%) | ||
| Baseline mean |
8.0 |
7.9 |
| Change from baseline [adjusted mean (SE)] a |
-0.9 (0.1) |
-0.8 (0.1) |
| Difference from sitagliptin [adjusted mean] (95% CI) |
0.1 (-0.1, 0.2) b |
|
| Proportion of patients (%) achieving HbA1c < 7% c |
45% |
40% |
| FPG (mg/dL) | ||
| Baseline mean |
180 |
176 |
| Change from baseline [adjusted mean (SE)] d |
-26 (2) |
-31 (2) |
| Difference from sitagliptin [adjusted mean] (95% CI) |
-5 (-11, 1) |
|
SE: Standard Error; CI: Confidence Interval; FPG: Fasting plasma glucose
aIntention to treat population. ANCOVA was used to analyze data using imputed values by return to baseline analysis for missing data at week 24 (6% and 2% for bexagliflozin and sitagliptin, respectively). The ANCOVA model included treatment, baseline HbA1c value and region. Non-inferiority is declared if the upper bound of the 95% confidence interval for the difference from sitagliptin lies below 0.35.
bNon-inferior
cCrude proportion using imputed HbA1c values for missing data at week 24 and averaged across multiply imputed datasets
dSame model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
14.4 Glycemic Control in Adults with Type 2 Diabetes Mellitus and Moderate Renal Impairment (Trial 5)
A total of 312 adults with inadequately controlled type 2 diabetes mellitus (HbA1c between 7.0% and 10.5%) and moderate renal impairment (eGFR between 30 and 60 mL/min/1.73 m 2) received Bexagliflozin or placebo in a double-blind, randomized, placebo-controlled trial (NCT02836873; referred to as Trial 5) to evaluate the efficacy of Bexagliflozin. In this trial, 77 Bexagliflozin-treated patients had an eGFR between 45 and 60 mL/min/1.73 m 2and 74 Bexagliflozin-treated patients had an eGFR between 30 and 45 mL/min/1.73 m 2. At baseline, nearly all patients (96%) were treated with one or more antidiabetic medications including metformin (37%), insulin (58%), sulfonylureas (11%) and dipeptidyl peptidase 4 (DPP-4) inhibitors (21%). The mean age of the population was 70 years and 29% of the patients were older than 75 years of age. Sixty-three percent (63%) were male and 55% were White, 38% were Asian, 5% were Black, and 2% were other races. Eight percent (8%) were Hispanic/Latino. The mean duration of type 2 diabetes mellitus was 16 years, the mean HbA1c at baseline was 8.0% and the mean eGFR was 45 mL/min/1.73 m 2.
At week 24, treatment with Bexagliflozin provided a statistically significant reduction in HbA1c compared to placebo ( Table 9).
Table 9. Glycemic Results from a 24-Week Placebo-Controlled Trial that Evaluated Bexagliflozin as a Therapy Added to Standard of Care Regimens for Adults with Type 2 Diabetes Mellitus and eGFR between 30 and 60 mL/min/1.73 m 2(Trial 5)
|
Placebo N = 155 |
Bexagliflozin N = 157 |
|
| HbA1c (%) | ||
| Baseline mean |
7.9 |
8.0 |
| Change from baseline [adjusted mean (SE)] a |
-0.3 (0.1) |
-0.6 (0.1) |
| Difference from placebo [adjusted mean] (95% CI) |
-0.3 (-0.4, -0.1) * |
|
| Proportion of patients (%) achieving HbA1c < 7% b |
22% |
33% |
| FPG (mg/dL) | ||
| Baseline mean |
155 |
156 |
| Change from baseline [adjusted mean (SE)] c |
-8 (3) |
-22 (3) |
| Difference from placebo [adjusted mean] (95% CI) |
-14 (-23, -5) |
|
SE: Standard Error; CI: Confidence Interval; FPG: Fasting plasma glucose
*Statistically significant (multiplicity adjusted one-sided p-value < 0.025)
aIntention to treat population. ANCOVA was used to analyze data using imputed values by return to baseline analysis for missing data at week 24 (3% and 5% for bexagliflozin and placebo, respectively). The ANCOVA model included treatment, region, screening anti-diabetic treatment regimen (insulin treated or other), baseline eGFR (< 45 or ≥ 45 mL/min/1.73m
2) and baseline HbA1c value.
bCrude proportion using imputed HbA1c values for missing data at week 24 and averaged across multiply imputed datasets
cSame model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
Body Weight Results
The mean baseline body weight in patients with baseline BMI ≥ 25 kg/m
2was 89 kg in both the placebo (N = 122) and Bexagliflozin (N = 125) groups. The mean changes from baseline to Week 24 in this population were -0.4 kg and -2.1 kg in the placebo and Bexagliflozin groups, respectively. The difference from placebo (95% CI) for Bexagliflozin was -1.7 kg (-2.4, -1.0).
14.5 Glycemic Control and Major Cardiovascular Events (MACE) in Adults with Type 2 Diabetes Mellitus and Increased Risk for Cardiovascular Disease (Trial 6)
The efficacy of Bexagliflozin was assessed in a multicenter, randomized, double-blind, placebo-controlled trial (NCT02558296; referred to as Trial 6) of adults with inadequately controlled type 2 diabetes mellitus (HbA1c between 7% and 11%) who had either established CVD (including a history of atherosclerotic vascular disease or a history of heart failure) or multiple risk factors for CVD. There were no restrictions on background antihyperglycemic medication use, aside from treatment with an SGLT2 inhibitor. After a single-blind, 2-week, placebo run-in period, 1,701 patients were randomized to receive placebo (N = 567) or Bexagliflozin 20 mg (N = 1,134) orally once daily.
At baseline, nearly all patients (99.4%) were treated with one or more antidiabetic medications including metformin (77%), insulin (53%), sulfonylureas (40%), DPP-4 inhibitors (13%) and thiazolidinediones (3%). The mean age of the population was 64 years of age and 11% of the patients were older than 75 years of age. Seventy percent (70%) were male and 77% were White, 10% were Asian, 9% were American Indian or Alaskan Native, and 4% were Black. Fifteen percent (15%) were Hispanic/Latino. At baseline, the mean duration of diabetes was 15 years and the mean HbA1c was 8.3%. The mean baseline eGFR was 78 mL/min/1.73 m
2; 80% of patients had an eGFR > 60 mL/min/1.73 m
2and 20% of patients had an eGFR 45 to 60 mL/min/1.73 m
2.
Glycemic Response
Treatment with Bexagliflozin provided a statistically significant reduction in HbA1c at Week 24 compared to treatment with placebo (see
Table 10). In addition, Bexagliflozin provided a statistically significant reduction in HbA1c compared to placebo in a subset of patients using background insulin (N = 902, difference from placebo -0.5% [95% CI: -0.6, -0.4]) and in a subset of patients using background sulfonylureas (N = 313, difference from placebo -0.4% [95% CI:-0.6, -0.2]).
Table 10. Glycemic Results from a 24-Week Trial in Adults with Type 2 Diabetes Mellitus with Established CVD or Multiple CVD Risk Factors (Trial 6)
|
Placebo N = 567 |
Bexagliflozin N = 1133 |
|
| HbA1c (%) | ||
| Baseline mean |
8.3 |
8.3 |
| Change from baseline [adjusted mean (SE)] a |
-0.4 (0.04) |
-0.8 (0.03) |
| Difference from placebo [adjusted mean] (95% CI) |
-0.4 (-0.5, -0.4)* |
|
| Proportion of patients (%) achieving HbA1c < 7% b |
17% |
29% |
| FPG (mg/dL) | ||
| Baseline mean |
162 |
166 |
| Change from baseline [adjusted mean (SE)] c |
-4 (2) |
-23 (1) |
| Difference from placebo [adjusted mean] (95% CI) |
-20 (-24, -15) |
|
SE: Standard Error; CI: Confidence Interval; FPG: Fasting plasma glucose
*Statistically significant (multiplicity adjusted one-sided p-value < 0.025)
aIntention to treat population. ANCOVA was used to analyze data using imputed values by return to baseline analysis for missing data at week 24 (7% and 6% for bexagliflozin and placebo, respectively). The ANCOVA model included treatment, region, baseline eGFR category (< 60 or ≥ 60 mL/min/1.73m
2), baseline BMI category (< 25 or ≥ 25 kg/m
2), history of heart failure (yes or no), insulin use or not, and baseline HbA1c value.
bCrude proportion using imputed HbA1c values for missing data at week 24 and averaged across multiply imputed datasets
cSame model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
Body Weight and Systolic Blood Pressure Results
The mean baseline body weight in a subgroup of patients with baseline BMI ≥ 25 kg/m
2was 95 kg (N = 522) and 97 kg (N = 1047) in the placebo and Bexagliflozin groups, respectively. The mean changes from baseline to Week 24 were -0.3 kg and -2.7 kg in the placebo and Bexagliflozin groups, respectively. The difference from placebo (95% CI) for Bexagliflozin was -2.3 kg (-2.8, -1.9).
The mean baseline systolic blood pressure (SBP) in a subgroup of patients with baseline values ≥ 140 mmHg was 150 mmHg in the placebo (N = 215) and Bexagliflozin (N = 448) groups. The mean changes in SBP from baseline to Week 24 were -6.6 mmHg and -9.2 mmHg in the placebo and Bexagliflozin groups, respectively. The difference from placebo (95% CI) for Bexagliflozin was -2.7 mmHg (-5.2, -0.1).
Major Adverse Cardiovascular Events (MACE) Results
Trial 6 was used to assess the impact of Bexagliflozin on MACE (a composite of cardiovascular death, non-fatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina). The minimum treatment duration was 52 weeks (median duration 2.4 years). In this trial, the proportion of patients who experienced at least one MACE event was 10.1% (57/567) in the placebo group and 7.9% (89/1132) in the Bexagliflozin group (4.2 MACE events per 100 person-years for placebo and 3.3 MACE events per 100 person-years for Bexagliflozin). No increased risk for MACE was observed in the Bexagliflozin group compared to the control group [estimated hazard ratio of 0.77 (95% CI: 0.56, 1.08)]. The Bexagliflozin group was not superior to the placebo group in reducing MACE.
16 HOW SUPPLIED/STORAGE AND HANDLING
Bexagliflozin 20 mg tablets are blue, caplet-shaped, biconvex, bevel-edged, film-coated debossed with “2” and inverted “2” on one side.
Bottles of 30 tablets – NDC: 51407-826-30
Bottles of 90 tablets – NDC: 51407-826-90
Storage and Handling
Store from 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30 °C (59 °F to 86 °F)
[see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
Inform patients that Bexagliflozin can cause potentially fatal ketoacidosis and that type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are risk factors.
Educate all patients on precipitating factors (such as insulin dose reduction or missed insulin doses, infection, reduced caloric intake, ketogenic diet, surgery, dehydration, and alcohol abuse) and symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing). Inform patients that blood glucose may be normal even in the presence of ketoacidosis.
Advise patients that they may be asked to monitor ketones. If symptoms of ketoacidosis occur, instruct patients to discontinue Bexagliflozin and seek medical attention immediately
[see Warnings and Precautions (
5.1)]
.
Lower Limb Amputation
Inform patients of the potential for an increased risk of amputations with Bexagliflozin. Counsel patients on the importance of routine preventive foot care. Instruct patients to monitor for any new pain or tenderness, sores or ulcers, or infections involving the leg or foot and to seek medical advice immediately if such signs or symptoms develop
[see Warnings and Precautions (5.2)].
Volume Depletion
Inform patients that symptomatic hypotension may occur with Bexagliflozin and advise them to contact their healthcare provider if they experience such symptoms. Inform patients that dehydration may increase the risk of hypotension, and to maintain adequate fluid intake.
[see Warnings and Precautions (5.3)].
Serious Urinary Tract Infections
Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur
[see Warnings and Precautions (5.4)].
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Inform patients that the incidence of hypoglycemia may increase when Bexagliflozin is added to insulin and/or an insulin secretagogue. Educate patients or caregivers on the signs and symptoms of hypoglycemia
[see Warnings and Precautions (5.5)].
Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Inform patients that necrotizing infections of the perineum (Fournier’s Gangrene) have occurred with Bexagliflozin. Counsel patients to promptly seek medical attention if they develop pain or tenderness, redness or swelling of the genitals or the area from the genitals to the anus, accompanied by a fever above 100.4 °F or malaise
[see Warnings and Precautions (5.6)].
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infections. Advise them of treatment options and when to seek medical advice
[see Warnings and Precautions (
5.7)].
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
Inform male patients that yeast infections of the penis (
e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males and patients with prior infections. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice
[see Warnings and Precautions (5.7)].
Pregnancy
Advise pregnant patients and females of reproductive potential of the potential risk to a fetus with treatment with Bexagliflozin. Instruct patients to inform their healthcare provider if pregnant or planning to become pregnant
[see Use in Specific Populations (8.1)].
Lactation
Advise patients that breastfeeding is not recommended during treatment with Bexagliflozin
[see Use in Specific Populations (8.2)].
Laboratory Tests
Inform patients that their urine will test positive for glucose while taking Bexagliflozin due to its mechanism of action.
[see Drug Interactions (
7)]
Missed Dose
Instruct patients to take Bexagliflozin only as prescribed. If a dose is missed, it should be taken as soon as possible. Advise patients not to double their next dose.
Distributed by:
TheracosBio, LLC
225 Cedar Hill Street, Suite 200
Marlborough, MA 01752 USA
Marketed by:
GSMS, Inc.
Camarillo, CA USA 93012
MEDGUIDE SECTION
|
MEDICATION GUIDE Bexagliflozin tablets for oral use |
| What is the most important information I should know about Bexagliflozin?
Bexagliflozin can cause serious side effects, including:
|
What is Bexagliflozin?
|
Do not take Bexagliflozin if you:
|
Before you take Bexagliflozin, tell your healthcare provider about all of your medical conditions, including if you:
Bexagliflozin may affect the way other medicines work, and other medicines may affect how Bexagliflozin works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
How should I take Bexagliflozin?
|
| What are the possible side effects of Bexagliflozin?
BRENZAVVY may cause serious side effects, including:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
| How should I store Bexagliflozin?
Store Bexagliflozin at room temperature between 68 °F to 77 °F (20 °C to 25 °C). Keep Bexagliflozin and all medicines out of the reach of children. |
| General information about the safe and effective use of Bexagliflozin.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Bexagliflozin for a condition for which it was not prescribed. Do not give Bexagliflozin to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Bexagliflozin that is written for health professionals. |
|
What are the ingredients in Bexagliflozin?
Brands listed are the trademarks of their respective owners. Marketed by: GSMS, Inc. Camarillo, CA USA 93012
|
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 09/2023
| BRENZAVVY
bexagliflozin tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Golden State Medical Supply, Inc. (603184490) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Golden State Medical Supply, Inc. | 603184490 | relabel(51407-826) , repack(51407-826) | |
Trademark Results [Brenzavvy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRENZAVVY 88854771 not registered Live/Pending |
Theracos Sub, LLC 2020-03-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
