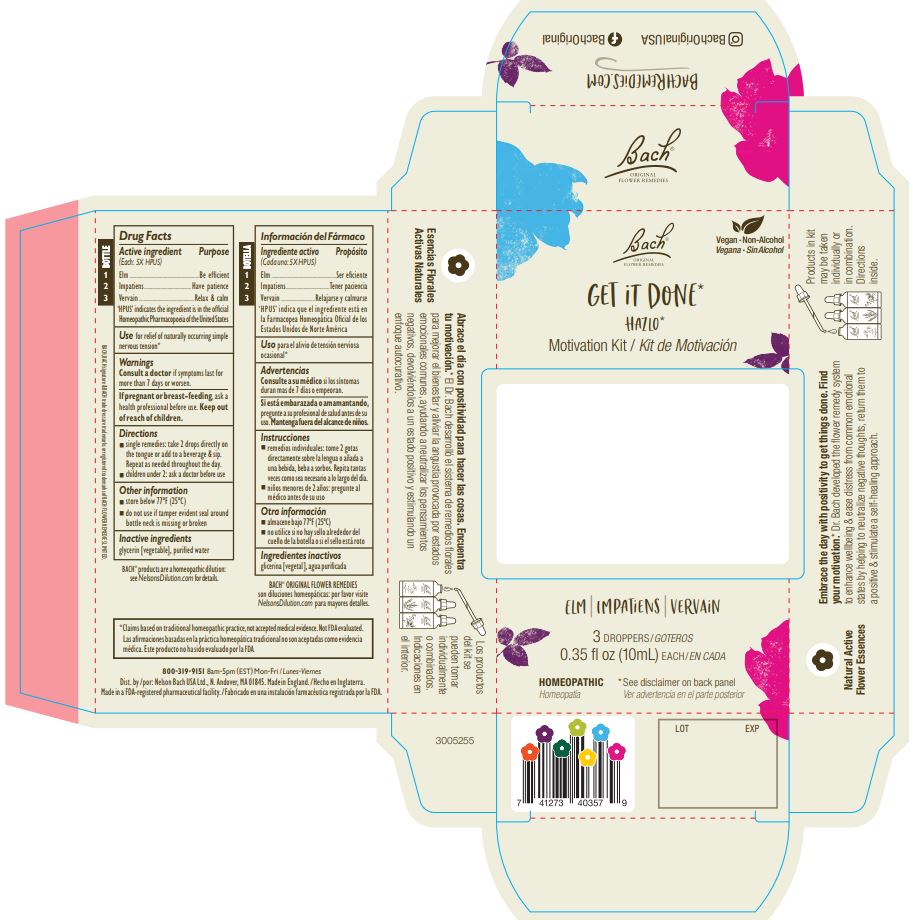
BACH GET IT DONE MOTIVATION KIT- elm, impatiens, vervain kit
Bach Get it Done Motivation Kit by
Drug Labeling and Warnings
Bach Get it Done Motivation Kit by is a Homeopathic medication manufactured, distributed, or labeled by Nelson Bach USA Limited, A. Nelson & Co. Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active ingredient (Each 5X HPUS)........... Purpose
Bottle 1: Elm...............................................Be efficient
Bottle 2: Impatiens......................................Have patience
Bottle 3: Vervain.........................................Relax & calm
'HPUS' indicates ingredients are in the official Homeopathic Pharmacopoeia of the United States
- Use
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACH GET IT DONE MOTIVATION KIT
elm, impatiens, vervain kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57687-314 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57687-314-30 1 in 1 CARTON 11/01/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, DROPPER 10 mL Part 2 1 BOTTLE, DROPPER 10 mL Part 3 1 BOTTLE, DROPPER 10 mL Part 1 of 3 BACH ORIGINAL FLOWER REMEDIES IMPATIENS
impatiens glandulifera solutionProduct Information Item Code (Source) NDC: 57687-217 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMPATIENS GLANDULIFERA FLOWER (UNII: AJU5O1A5ZV) (IMPATIENS GLANDULIFERA FLOWER - UNII:AJU5O1A5ZV) IMPATIENS GLANDULIFERA FLOWER 5 [hp_X] in 0.095 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57687-217-10 1 in 1 CARTON 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2023 Part 2 of 3 BACH ORIGINAL FLOWER REMEDIES VERVAIN
verbena officinalis solutionProduct Information Item Code (Source) NDC: 57687-230 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VERBENA OFFICINALIS FLOWERING TOP (UNII: 311PNK9CDZ) (VERBENA OFFICINALIS FLOWERING TOP - UNII:311PNK9CDZ) VERBENA OFFICINALIS FLOWERING TOP 5 [hp_X] in 0.095 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57687-230-10 1 in 1 CARTON 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2023 Part 3 of 3 BACH ORIGINAL FLOWER REMEDIES ELM
ulmus procera solutionProduct Information Item Code (Source) NDC: 57687-210 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ULMUS PROCERA FLOWERING TWIG (UNII: J3O020I532) (ULMUS PROCERA FLOWERING TWIG - UNII:J3O020I532) ULMUS PROCERA FLOWERING TWIG 5 [hp_X] in 0.095 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57687-210-10 1 in 1 CARTON 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2023 Labeler - Nelson Bach USA Limited (877720193) Registrant - A. Nelson & Co. Limited (221471234) Establishment Name Address ID/FEI Business Operations A. Nelson & Co. Limited 221471234 manufacture(57687-314)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.