VECAMYL- mecamylamine hydrochloride tablet
Vecamyl by
Drug Labeling and Warnings
Vecamyl by is a Prescription medication manufactured, distributed, or labeled by Vyera Pharmaceuticals, LLC, Nexgen Pharma, Inc., LGM Pharma Solutions, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
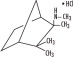
Mecamylamine HCl is a potent, oral antihypertension agent and ganglion blocker, and is a secondary amine. It is N,2,3,3-tetramethyl-bicyclo [2.2.1] heptan- 2 -amine hydrochloride. Its empirical formula is C11H21N HCl and its structural formula is:
It is a white, odorless, or practically odorless, crystalline powder, is highly stable, soluble in water and has a molecular weight of 203.75. Mecamylamine HCl is supplied as tablets for oral use, each containing 2.5 mg mecamylamine HCl. Inactive ingredients are calcium phosphate, D&C Yellow 10, FD&C Yellow 6, lactose, magnesium stearate, cornstarch, and talc.
-
CLINICAL PHARMACOLOGY
Mecamylamine HCl reduces blood pressure in both normotensive and hypertensive individuals. It has a gradual onset of action (1/2 to 2 hours) and a long-lasting effect (usually 6 to 12 hours or more). A small oral dosage often produces a smooth and predictable reduction of blood pressure. Although this antihypertensive effect is predominantly orthostatic, the supine blood pressure is also significantly reduced.
Pharmacokinetics and Metabolism
Mecamylamine HCl is almost completely absorbed from the gastrointestinal tract, resulting in consistent lowering of blood pressure in most patients with hypertensive cardiovascular disease. Mecamylamine HCl is excreted slowly in the urine in the unchanged form. The rate of its renal elimination is influenced markedly by urinary pH. Alkalinization of the urine reduces, and acidification promotes, renal excretion of mecamylamine.
Mecamylamine HCl crosses the blood-brain and placental barriers.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Mecamylamine HCl should not be used in mild, moderate, labile hypertension and may prove unsuitable in uncooperative patients. It is contraindicated in coronary insufficiency or recent myocardial infarction.
Mecamylamine HCl should be given with great discretion, if at all, when renal insufficiency is manifested by a rising or elevated BUN. The drug is contraindicated in uremia. Patients receiving antibiotics and sulfonamides should generally not be treated with ganglion blockers. Other contraindications are glaucoma, organic pyloric stenosis or hypersensitivity to the product.
-
WARNINGS
Mecamylamine, a secondary amine, readily penetrates into the brain and thus may produce central nervous system effects. Tremor, choreiform movements, mental aberrations, and convulsions may occur rarely. These have occurred most often when large doses of Mecamylamine HCl were used, especially in patients with cerebral or renal insufficiency.
When ganglion blockers or other potent antihypertensive drugs are discontinued suddenly, hypertensive levels return. In patients with malignant hypertension and others, this may occur abruptly and may cause fatal cerebral vascular accidents or acute congestive heart failure. When Mecamylamine HCl is withdrawn, this should be done gradually and other antihypertensive therapy usually must be substituted. On the other hand, the effects of Mecamylamine HCl sometimes may last from hours to days after therapy is discontinued.
-
PRECAUTIONS
General
The patient's condition should be evaluated carefully, particularly as to renal and cardiovascular function. When renal, cerebral, or coronary blood flow is deficient, any additional impairment, which might result from added hypotension, must be avoided. The use of Mecamylamine HCl in patients with marked cerebral and coronary arteriosclerosis or after a recent cerebral accident requires caution.
The action of Mecamylamine HCl may be potentiated by excessive heat, fever, infection, hemorrhage, pregnancy, anesthesia, surgery, vigorous exercise, other antihypertensive drugs, alcohol, and salt depletion as a result of diminished intake or increased excretion due to diarrhea, vomiting, excessive sweating, or diuretics.
During therapy with Mecamylamine HCl, sodium intake should not be restricted but, if necessary, the dosage of the ganglion blocker must be adjusted.
Since urinary retention may occur in patients on ganglion blockers, caution is required in patients with prostatic hypertrophy, bladder neck obstruction, and urethral stricture.
Frequent loose bowel movements with abdominal distention and decreased borborygmi may be the first signs of paralytic ileus. If these are present, Mecamylamine HCl should be discontinued immediately and remedial steps taken.
Information for Patients
Mecamylamine HCl may cause dizziness, lightheadedness, or fainting, especially when rising from a lying or sitting position. This effect may be increased by alcoholic beverages, exercise, or during hot weather. Getting up slowly may help alleviate such a reaction.
Drug Interactions
Patients receiving antibiotics and sulfonamides generally should not be treated with ganglion blockers.
The action of Mecamylamine HCl may be potentiated by anesthesia, other antihypertensive drugs and alcohol.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the effects upon fertility, mutagenic or carcinogenic potential of Mecamylamine HCl.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with Mecamylamine HCl. It is not known whether Mecamylamine HCl can cause fetal harm when given to a pregnant woman or can affect reproductive capacity. Mecamylamine HCl should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
The following adverse reactions have been reported and within each category are listed in order of decreasing severity.
Gastrointestinal: Ileus, constipation (sometimes preceded by small, frequent liquid stools), vomiting, nausea, anorexia, glossitis and dryness of mouth.
Cardiovascular: Orthostatic dizziness and syncope, postural hypotension.
Nervous System/Psychiatric: Convulsions, choreiform movements, mental aberrations, tremor, and paresthesias (see WARNINGS).
Respiratory: Interstitial pulmonary edema and fibrosis.
Urogenital: Urinary retention, impotence, decreased libido.
Special Senses: Blurred vision, dilated pupils.
Miscellaneous: Weakness, fatigue, sedation.
-
OVERDOSAGE
Signs of overdosage include: hypotension (which may progress to peripheral vascular collapse), postural hypotension, nausea, vomiting, diarrhea, constipation, paralytic ileus, urinary retention, dizziness, anxiety, dry mouth, mydriasis, blurred vision, or palpitations. A rise in intraocular pressure may occur.
Pressor amines may be used to counteract excessive hypotension. Since patients being treated with ganglion blockers are more than normally reactive to pressor amines, small doses of the latter are recommended to avoid excessive response.
The oral LD50 of Mecamylamine HCl in the mouse is 92 mg/kg.
-
DOSAGE AND ADMINISTRATION
Therapy is usually started with one 2.5 mg tablet of Mecamylamine HCl twice a day. This initial dosage should be modified by increments of one 2.5 mg tablet at intervals of not less than 2 days until the desired blood pressure response occurs (the criterion being a dosage just under that which causes signs of mild postural hypotension).
The average total daily dosage of Mecamylamine HCl is 25 mg, usually in three divided doses. However, as little as 2.5 mg daily may be sufficient to control hypertension in some patients. A range of two to four or even more doses may be required in severe cases when smooth control is difficult to obtain. In severe or urgent cases, larger increments at smaller intervals may be needed. Partial tolerance may develop in certain patients, requiring an increase in the daily dosage of Mecamylamine HCl.
Administration of Mecamylamine HCl after meals may cause a more gradual absorption and smoother control of excessively high blood pressure. The timing of doses in relation to meals should be consistent. Since the blood pressure response to antihypertensive drugs is increased in the early morning, the larger dose should be given at noontime and perhaps in the evening. The morning dose, as a rule, should be relatively small and in some instances may even be omitted.
The initial regulation of dosage should be determined by blood pressure readings in the erect position at the time of maximal effect of the drug, as well as by other signs and symptoms of orthostatic hypotension.
The effective maintenance dosage should be regulated by blood pressure readings in the erect position and by limitation of dosage to that which causes slight faintness or dizziness in this position. If the patient or a relative can use a sphygmomanometer, instructions may be given to reduce or omit a dose if readings fall below a designated level or if faintness or lightheadedness occurs. However, no change should be instituted without the knowledge of the physician.
Close supervision and education of the patient, as well as critical adjustment of dosage, are essential to successful therapy.
Other Antihypertensive Agents
When Mecamylamine HCl is given with other antihypertensive drugs, the dosage of these other agents, as well as that of Mecamylamine HCl, should be reduced to avoid excessive hypotension. However, thiazides should be continued in their usual dosage, while that of Mecamylamine HCl is decreased by at least 50 percent.
-
HOW SUPPLIED
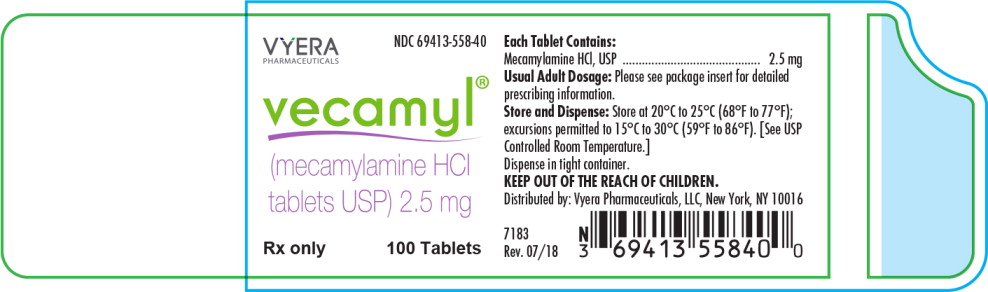
Vecamyl® (MECAMYLAMINE Hydrochloride Tablets, USP) are slightly yellow, round, compressed tablets, coded MP on one side and 2.5 on the other side. They are supplied as follows:
NDC 69413-558-40 in bottles of 100.
STORAGE CONDITION
Store at 20°C to 25°C (68°F to 77°F) excursions permitted to 15°C to 30°C (59°F to 86°F)
[see USP Controlled Room Temperature.]Distributed by:
Vyera Pharmaceuticals, LLC
New York, NY 10016
7183
Rev. 07/18 -
Principal Display Panel - Vecamyl Bottle Label
VYERA
PHARMACEUTICALS
NDC: 69413-558-41
vecamyl®
(mecamylamine HCl
tablets USP) 2.5 mg
Rx only
100 Tablets -
INGREDIENTS AND APPEARANCE
VECAMYL
mecamylamine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69413-558 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECAMYLAMINE HYDROCHLORIDE (UNII: 4956DJR58O) (MECAMYLAMINE - UNII:6EE945D3OK) MECAMYLAMINE HYDROCHLORIDE 2.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) Talc (UNII: 7SEV7J4R1U) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (YELLOW) Score no score Shape ROUND (ROUND) Size 6mm Flavor Imprint Code MP;2;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69413-558-40 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/19/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204054 03/19/2013 Labeler - Vyera Pharmaceuticals, LLC (079604693) Registrant - Nexgen Pharma, Inc. (048488621) Establishment Name Address ID/FEI Business Operations Nexgen Pharma, Inc. 160356114 MANUFACTURE(69413-558) , ANALYSIS(69413-558) , PACK(69413-558) , LABEL(69413-558)
Trademark Results [Vecamyl]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VECAMYL 85278488 4358457 Live/Registered |
PHOENIXUS AG 2011-03-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.