SODIUM CITRATE 4% W/V ANTICOAGULANT- trisodium citrate dihydrate injection, solution
Sodium Citrate 4% w/v Anticoagulant by
Drug Labeling and Warnings
Sodium Citrate 4% w/v Anticoagulant by is a Prescription medication manufactured, distributed, or labeled by Terumo BCT, Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP safely and effectively. See full prescribing information for SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP.
SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP
Sterile Fluid
Polyvinyl Chloride (PVC) Bag
Initial U.S. Approval: 1978INDICATIONS AND USAGE
SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP is intended for use only for the anticoagulation of whole blood as part of automated apheresis procedures. (1)
DOSAGE AND ADMINISTRATION
- SODIUM CITRATE 4% ANTICOAGULANT SOLUTION USP is added to tubing sets during apheresis procedures. (2)
- SODIUM CITRATE 4% ANTICOAGULANT SOLUTION USP may only be used with apheresis devices. For instructions on the use of the solution see the apheresis device operator's manual. (2.1)
- Follow the directions for connecting the SODIUM CITRATE 4% ANTICOAGULANT SOLUTION USP bag to the apheresis system. (2.2)
DOSAGE FORMS AND STRENGTHS
- 250 mL sterile fluid in a PVC bag. (3)
CONTRAINDICATIONS
- DO NOT INFUSE SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP DIRECTLY TO THE DONOR. (4)
WARNINGS AND PRECAUTIONS
- Verify that the SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP has been securely attached to the Anticoagulant (AC) line on the system tubing set. Use aseptic technique throughout all procedures to ensure donor safety and quality. (5)
ADVERSE REACTIONS
Citrate reactions or toxicity may occur with the infusion and return of blood containing citrate anticoagulant. The recipient of the blood containing citrate should be monitored for the signs and symptoms of citrate toxicity. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Terumo BCT, Inc. at 1-877-339-4228 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
ANTICOAGULANT SOLUTION has not been studied in controlled clinical trials with specific populations. (7)
Revised: 8/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION is intended for use only for the anticoagulation of whole blood as part of automated apheresis procedures. [See Dosage and Administration (2).]
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP is added to tubing sets during apheresis procedures. The solution is connected the tubing set in an apheresis collection. The recommended dose is determined by the apheresis device and metered into the tubing set by the apheresis device. It is not intended for direct intravenous infusion.
For instructions on the use of the solution with the apheresis device and tubing set, see the device operator's manual.
2.2 Administration
- Ensure solution is the SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP and is within the expiration date.
- Inspect the bag. Do not use if the container is damaged, leaking or if there is any visible sign of deterioration.
- Use only if solution is clear and free of particulate matter.
- Protect from sharp objects.
Directions for connecting the SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP bag to the apheresis device.
At the prompt to connect anticoagulant to the apheresis device tubing set:
- Remove the overwrap by pulling down at the notch, and remove the SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP bag.
- Before use, perform the following checks [See Warnings and Precautions (5).]:
- Check for leaks by gently squeezing the bag. If leaks are found, discard the bag.
- Ensure that the solution is the SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP and is within the expiration date.
- Inspect the solution in good light. Bags showing cloudiness, haze, or particulate matter should not be used.
- Remove the protective cap from the port on the bag.
- Connect the bag to the apheresis device tubing set using aseptic technique and hang the solution.
- Proceed according to the apheresis device operator's manual.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
Citrate reactions or toxicity may occur with the infusion of blood products to patients and return of blood containing citrate anticoagulant to donors. The recipient of the blood containing citrate should be monitored for the signs and symptoms of citrate toxicity. The signs and symptoms of citrate toxicity begin with paresthesia, a "tingling" sensation around the mouth or in the extremities, followed by severe reactions that are characterized by hypotension and possible cardiac arrhythmia. Citrate toxicity may occur more frequently in patients who are hypothermic, have impaired liver or renal function, or have low calcium levels because of an underlying disease.
- 8 USE IN SPECIFIC POPULATIONS
-
11 DESCRIPTION
SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP is designed to be metered by an apheresis device in apheresis procedures, to prevent platelet activation and coagulation as blood moves throughout the extracorporeal unit (tubing set) in an apheresis procedure.
The solution is sterile and non-pyrogenic, and it contains no bacteriostatic or antimicrobial agents.
The formulas of the active ingredients are provided in Table 1.
Table 1: Active Ingredients Ingredients Molecular Formula Molecular Weight Sodium Citrate Dihydrate C6H9Na3O9 294.10 Water for Injection H2O 18.00 Each 100 mL of SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP contains: Sodium Citrate (dihydrate) 4.0 g; and 100mL Water for Injection (pH adjusted with citric acid).
The PVC bag is not made with natural rubber latex.
The bag is made from a multilayered film. It contains materials that have been tested to demonstrate the suitability of the container for storing pharmaceutical solutions. The bag is nontoxic and biologically inert. The bag-solution unit is a closed system and is not dependent upon entry of external air during administration. The bag is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SODIUM CITRATE 4% W/V ANTICOAGULANT SOLUTION USP acts as an extracorporeal anticoagulant by binding the free calcium in the blood. Calcium is a necessary co-factor to several steps in the clotting cascade. The following ingredients are key components of the solution:
- Citric acid for pH regulation
- Sodium Citrate anticoagulant
This solution has no pharmacological effect.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Carton Label
Sodium Citrate 4% w/v
Anticoagulant Solution USPCatalog # 40881
30 x 250 mL units
NDC: 14537-881-25For use only with automated apheresis procedures.
See apheresis device operator's manual for complete instructions.
Read the package insert before application.
Sterile. Non-pyrogenic. Sterilized with steam.
Caution: Do not use unless the solution is clear and the container is intact.
Single use container. Discard any unused product. Not for direct
intravenous infusion. Rx Only.Recommended storage:
Up to 25 °C.
Protect from freezing.Each 100 mL contains:
Sodium Citrate Dihydrate
4.0 g
Water for Injection to
(pH adjusted with citric acid)
100 mL
Approximate Millimoles:
Sodium Citrate
13.8Manufactured by Terumo BCT, Inc.
10811 W. Collins Ave., Lakewood CO 80215, USA777962-240
TERUMOBCTLot
Expiry Date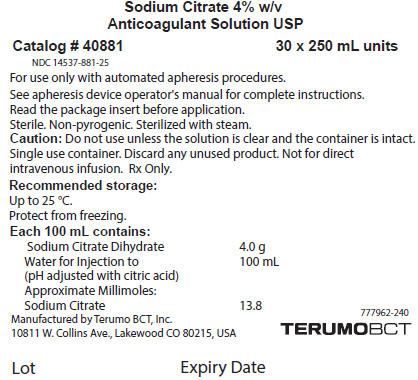
-
INGREDIENTS AND APPEARANCE
SODIUM CITRATE 4% W/V ANTICOAGULANT
trisodium citrate dihydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 14537-881 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 4 g in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Citric Acid monohydrate (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14537-881-25 30 in 1 CARTON 1 250 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA BA125608 06/26/2018 Labeler - Terumo BCT, Ltd (233649834) Establishment Name Address ID/FEI Business Operations Terumo BCT, Ltd 233649834 MANUFACTURE(14537-881) , ANALYSIS(14537-881) , STERILIZE(14537-881) , LABEL(14537-881)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.