Zantanew Tab by Cho A Pharm Co.,Ltd
Zantanew Tab by
Drug Labeling and Warnings
Zantanew Tab by is a Otc medication manufactured, distributed, or labeled by Cho A Pharm Co.,Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZANTANEW TAB- zantanew tab tablet
Cho A Pharm Co.,Ltd
----------
Ask a doctor or pharmacist before use if you are taking a prescription
drug. Antacids may interact with certain prescription drugs.
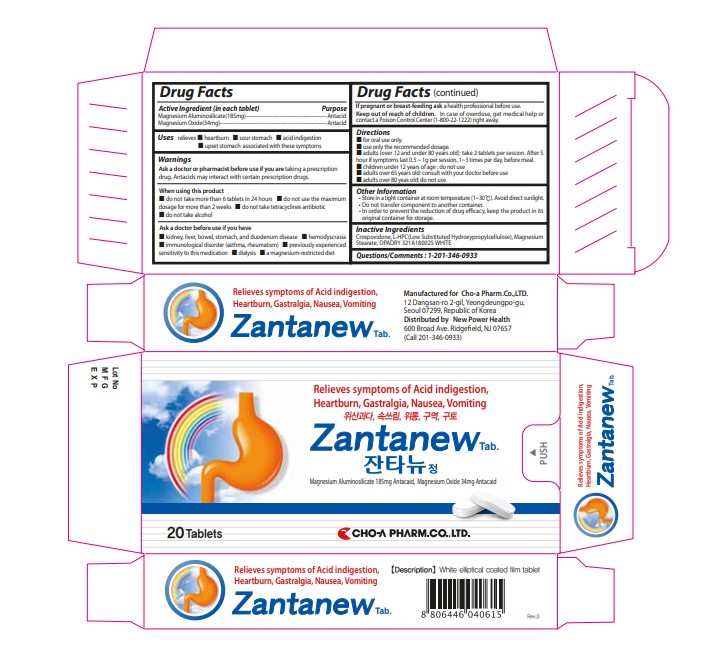
■ children under 12 years of age : do not use
■ adults over 80 yeas old: do not use
■ for oral use only

| ZANTANEW TAB
zantanew tab tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cho A Pharm Co.,Ltd (688056831) |
| Registrant - Cho A Pharm Co.,Ltd (688056831) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cho A Pharm Co.,Ltd | 688056831 | manufacture(58354-201) | |
Revised: 6/2024
Document Id: 1b8bcfa8-7f8c-8423-e063-6394a90a58dd
Set id: 07806e4b-9125-a533-e063-6394a90a16fc
Version: 5
Effective Time: 20240623
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.