AVANDAMET- rosiglitazone maleate and metformin hydrochloride tablet, film coated
AVANDAMET by
Drug Labeling and Warnings
AVANDAMET by is a Prescription medication manufactured, distributed, or labeled by Physicians Total Care, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AVANDAMET safely and effectively. See full prescribing information for AVANDAMET.
AVANDAMET (rosiglitazone maleate and metformin hydrochloride) Tablets
Initial U.S. Approval: 2002WARNINGS
See full prescribing information for complete boxed warning.
Rosiglitazone maleate: CONGESTIVE HEART FAILURE AND MYOCARDIAL INFARCTION
- Thiazolidinediones, including rosiglitazone, cause or exacerbate heart failure in some patients (5.2). After initiation of AVANDAMET, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction must be considered. (5.2)
- AVANDAMET is not recommended in patients with symptomatic heart failure. Initiation of AVANDAMET in patients with established NYHA Class III or IV heart failure is contraindicated. (4, 5.2)
- A meta-analysis of 52 clinical trials (mean duration 6 months; 16,995 total patients), most of which compared rosiglitazone to placebo, showed rosiglitazone to be associated with a statistically significant increased risk of myocardial infarction. Three other trials (mean duration 46 months; 14,067 total patients), comparing rosiglitazone to some other approved oral antidiabetic agents or placebo, showed a statistically non-significant increased risk of myocardial infarction and a statistically non-significant decreased risk of death. There have been no clinical trials directly comparing cardiovascular risk of rosiglitazone and ACTOS® (pioglitazone, another thiazolidinedione), but in a separate trial, pioglitazone (when compared to placebo) did not show an increased risk of myocardial infarction or death. (5.3)
Metformin hydrochloride: LACTIC ACIDOSIS
- Lactic acidosis can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic insufficiency, renal impairment and acute congestive heart failure. (5.1)
- Symptoms include malaise, myalgias, respiratory distress, increasing somnolence and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap and elevated blood lactate. (5.1)
- If acidosis is suspected, discontinue AVANDAMET and hospitalize the patient immediately. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
AVANDAMET is a combination antidiabetic product containing a thiazolidinedione and a biguanide. After consultation with a healthcare professional who has considered and advised the patient of the risks and benefits of rosiglitazone, this drug is indicated as an adjunct to diet and exercise to improve glycemic control when treatment with both rosiglitazone and metformin is appropriate in adults with type 2 diabetes mellitus who either are: (1)
- already taking rosiglitazone, or
- not already taking rosiglitazone and are unable to achieve glycemic control on other diabetes medications and, in consultation with their healthcare provider, have decided not to take pioglitazone (ACTOS) or pioglitazone-containing products (ACTOPLUS MET®, ACTOPLUS MET XR®, DUETACT®) for medical reasons. (1)
Other Important Limitations of Use: (1)
- Should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis. (1)
- Coadministration with insulin is not recommended. (1, 5.2, 5.3)
DOSAGE AND ADMINISTRATION
- Individualize the starting dose based on the patient’s current regimen. (2.1)
- Dose increases should be accompanied by careful monitoring for adverse events related to fluid retention. (2.1)
- Give in divided doses with meals with gradual dose escalation to reduce the gastrointestinal side effects. (2.2)
- Do not exceed the maximum recommended daily dose of 8 mg rosiglitazone and 2,000 mg metformin. (2.3)
- Do not initiate if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels. (2.4)
DOSAGE FORMS AND STRENGTHS
Oval, film-coated tablets containing rosiglitazone/metformin hydrochloride: 2 mg/500 mg, 4 mg/500 mg, 2 mg/1,000 mg, and 4 mg/1,000 mg (3) (3)
CONTRAINDICATIONS
- Initiation in patients with established NYHA Class III or IV heart failure. (4)
- Use in significant renal disease or renal dysfunction. (4)
- Use in acute or chronic metabolic acidosis. (4)
- Use in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials. (4, 5.1)
WARNINGS AND PRECAUTIONS
- Fluid retention, which may exacerbate or lead to heart failure, may occur. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk of other cardiovascular effects. (5.2)
- Increased risk of myocardial infarction has been observed in a meta-analysis of 52 clinical trials of rosiglitazone (incidence rate 0.4% versus 0.3%). (5.3)
- Coadministration with insulin is not recommended. (1, 5.2, 5.3)
- Assess renal function before starting therapy and at least annually. (5.1)
- Avoid use in patients with evidence of hepatic disease. (2.4, 5.1)
- Warn patients against excessive alcohol intake. (5.1)
- Promptly evaluate patients who develop laboratory abnormalities or clinical illness for evidence of ketoacidosis or lactic acidosis. (5.1)
- Dose-related edema (5.4), weight gain (5.5), and anemia (5.9) may occur.
- Macular edema has been reported. (5.7)
- Increased incidence of bone fracture. (5.8)
- Measure hematologic parameters annually. (5.9)
ADVERSE REACTIONS
The most common adverse reactions (≥10%) include nausea/vomiting, diarrhea, headache, and dyspepsia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Inhibitors of CYP2C8 (e.g., gemfibrozil) may increase rosiglitazone levels. (7.1)
- Inducers of CYP2C8 (e.g., rifampin) may decrease rosiglitazone levels. (7.1)
- Cationic drugs eliminated by renal tubular secretion; use with caution. (7.2)
USE IN SPECIFIC POPULATIONS
- Do not use during pregnancy. No human or animal data. (8.1)
- Safety and effectiveness in children under 18 years have not been established. (8.4)
- Because reduced renal function is associated with increasing age, use with caution in elderly patients. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2012
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNINGS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Starting Dose
2.2 Dose Titration
2.3 Maximum Dose
2.4 Specific Patient Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis
5.2 Cardiac Failure
5.3 Major Adverse Cardiovascular Events
5.4 Edema
5.5 Weight Gain
5.6 Hepatic Effects
5.7 Macular Edema
5.8 Fractures
5.9 Hematologic Effects
5.10 Vitamin B12 Levels
5.11 Diabetes and Blood Glucose Control
5.12 Ovulation
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Laboratory Abnormalities
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Metabolized by Cytochrome P450
7.2 Cationic Drugs
7.3 Drugs That Produce Hyperglycemia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Drug-Drug Interactions
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Patient Advice
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNINGS
Rosiglitazone maleate: CONGESTIVE HEART FAILURE AND MYOCARDIAL INFARCTION
- Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients [see Warnings and Precautions (5.2)]. After initiation of AVANDAMET, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of AVANDAMET must be considered.
- AVANDAMET is not recommended in patients with symptomatic heart failure. Initiation of AVANDAMET in patients with established NYHA Class III or IV heart failure is contraindicated. [See Contraindications (4) and Warnings and Precautions (5.2).]
- A meta-analysis of 52 clinical trials (mean duration 6 months; 16,995 total patients), most of which compared rosiglitazone to placebo, showed rosiglitazone to be associated with an increased risk of myocardial infarction. Three other trials (mean duration 46 months; 14,067 total patients), comparing rosiglitazone to some other approved oral antidiabetic agents or placebo, showed a statistically non-significant increased risk of myocardial infarction, and a statistically non-significant decreased risk of death. There have been no clinical trials directly comparing cardiovascular risk of rosiglitazone and ACTOS® (pioglitazone, another thiazolidinedione), but in a separate trial, pioglitazone (when compared to placebo) did not show an increased risk of myocardial infarction or death. [See Warnings and Precautions (5.3).]
Metformin hydrochloride: LACTIC ACIDOSIS
- Lactic acidosis is a rare, but serious complication that can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic insufficiency, renal impairment, and acute congestive heart failure. [See Warnings and Precautions (5.1).]
- Symptoms include malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap and elevated blood lactate. [See Warnings and Precautions (5.1).]
- If acidosis is suspected, discontinue AVANDAMET and hospitalize the patient immediately [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
After consultation with a healthcare professional who has considered and advised the patient of the risks and benefits of rosiglitazone, AVANDAMET® is indicated as an adjunct to diet and exercise to improve glycemic control when treatment with both rosiglitazone and metformin is appropriate in adults with type 2 diabetes mellitus who either are:
- already taking rosiglitazone, or
- not already taking rosiglitazone and unable to achieve glycemic control on other diabetes medications and, in consultation with their healthcare provider, have decided not to take pioglitazone (ACTOS®) or pioglitazone-containing products (ACTOPLUS MET®, ACTOPLUS MET XR®, DUETACT®) for medical reasons.
- Other Important Limitations of Use:
- Due to its mechanism of action, rosiglitazone is active only in the presence of endogenous insulin. Therefore, AVANDAMET should not be used in patients with type 1 diabetes.
- Coadministration of AVANDAMET with insulin is not recommended [see Warnings and Precautions (5.2, 5.3)].
-
2 DOSAGE AND ADMINISTRATION
Prior to prescribing AVANDAMET, refer to Indications and Usage (1) for appropriate patient selection.
2.1 Starting Dose
AVANDAMET is generally given in divided doses with meals.
All patients should start the rosiglitazone component of AVANDAMET at the lowest recommended dose. Further increases in the dose of rosiglitazone should be accompanied by careful monitoring for adverse events related to fluid retention [see Boxed Warning and Warnings and Precautions (5.4)].
If therapy with a combination tablet containing rosiglitazone and metformin is considered appropriate for a patient with type 2 diabetes mellitus, then the selection of the dose of AVANDAMET should be based on the patient’s current doses of rosiglitazone and/or metformin.
To switch to AVANDAMET for patients currently treated with metformin, the usual starting dose of AVANDAMET is 4 mg rosiglitazone (total daily dose) plus the dose of metformin already being taken (see Table 1).
To switch to AVANDAMET for patients currently treated with rosiglitazone, the usual starting dose of AVANDAMET is 1,000 mg metformin (total daily dose) plus the dose of rosiglitazone already being taken (see Table 1).
When switching from combination therapy of rosiglitazone plus metformin as separate tablets, the usual starting dose of AVANDAMET is the dose of rosiglitazone and metformin already being taken.
Table 1. AVANDAMET Starting Dose for Patients Treated with Metformin and/or Rosiglitazone PRIOR THERAPY Usual AVANDAMET Starting Dose Total daily dose Tablet strength Number of tablets Metformina 1,000 mg/day 2 mg/500 mg 1 tablet twice a day 2,000 mg/day 2 mg/1,000 mg 1 tablet twice a day Rosiglitazone 4 mg/day 2 mg/500 mg 1 tablet twice a day 8 mg/day 4 mg/500 mg 1 tablet twice a day aFor patients on doses of metformin between 1,000 and 2,000 mg/day, initiation of AVANDAMET requires individualization of therapy.
2.2 Dose Titration
AVANDAMET is generally given in divided doses with meals, with gradual dose escalation. This reduces gastrointestinal side effects (largely due to metformin) and permits determination of the minimum effective dose for the individual patient.
Sufficient time should be given to assess adequacy of therapeutic response. FPG should be used initially to determine the therapeutic response to AVANDAMET. If additional glycemic control is needed, the daily dose of AVANDAMET may be increased by increments of 4 mg rosiglitazone and/or 500 mg metformin.
After an increase in metformin dosage, dose titration is recommended if patients are not adequately controlled after 1 to 2 weeks. After an increase in rosiglitazone dosage, dose titration is recommended if patients are not adequately controlled after 8 to 12 weeks.
2.3 Maximum Dose
The maximum recommended total daily dose of AVANDAMET is 8 mg rosiglitazone (taken as 4 mg twice daily) and 2,000 mg metformin (taken as 1,000 mg twice daily).
2.4 Specific Patient Populations
Renal Impairment: Any dosage adjustment should be based on a careful assessment of renal function. Generally, elderly, debilitated, and malnourished patients should not be titrated to the maximum dose of AVANDAMET. Monitoring of renal function is necessary to aid in prevention of metformin-associated lactic acidosis, particularly in the elderly [see Warnings and Precautions (5.1)].
Hepatic Impairment: Liver enzymes should be measured prior to initiating treatment with AVANDAMET. Therapy with AVANDAMET should not be initiated if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5X upper limit of normal at start of therapy). After initiation of AVANDAMET, liver enzymes should be monitored periodically per the clinical judgment of the healthcare professional [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
Geriatric: The initial and maintenance dosing of AVANDAMET should be conservative in patients with advanced age, due to the potential for decreased renal function in this population.
Pediatric: Safety and effectiveness of AVANDAMET in pediatric patients have not been established. AVANDAMET and rosiglitazone are not recommended for use in pediatric patients.
Pregnancy: AVANDAMET is not recommended for use in pregnancy.
-
3 DOSAGE FORMS AND STRENGTHS
Each film-coated oval tablet contains rosiglitazone as the maleate and metformin hydrochloride as follows:
- 2 mg/500 mg – pale pink, debossed with gsk on one side and 2/500 on the other
- 4 mg/500 mg – orange, debossed with gsk on one side and 4/500 on the other
- 2 mg/1,000 mg – yellow, debossed with gsk on one side and 2/1000 on the other
- 4 mg/1,000 mg – pink, debossed with gsk on one side and 4/1000 on the other
-
4 CONTRAINDICATIONS
- Initiation in patients with established New York Heart Association (NYHA) Class III or IV heart failure [see Boxed Warning].
- Use in patients with renal disease or renal dysfunction (e.g., as suggested by serum creatinine levels ≥1.5 mg/dL [males], ≥1.4 mg/dL [females], or abnormal creatinine clearance), which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia [see Warnings and Precautions (5.1)].
- Use in patients with acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
- Use in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because use of such products may result in acute alteration of renal function. AVANDAMET should be temporarily discontinued in these patients. [See Warnings and Precautions (5.1).]
-
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis
Incidence and Management: Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with AVANDAMET; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5 mcg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin is very low (approximately 0.03 cases/1,000 patient years of exposure, with approximately 0.015 fatal cases/1,000 patient years of exposure). Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking AVANDAMET and by use of the minimum effective dose of AVANDAMET. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Treatment with AVANDAMET should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, AVANDAMET should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, AVANDAMET should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking AVANDAMET, since alcohol potentiates the effects of metformin on lactate metabolism. In addition, AVANDAMET should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure.
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur. AVANDAMET should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose and, if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of AVANDAMET, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking AVANDAMET do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity or technical problems in sample handling.
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking AVANDAMET, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery [see Contraindications (4)].
Factors That May Predispose Patients to Lactic Acidosis: Assessment of Renal Function: Metformin is known to be substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of impairment of renal function. Thus, patients with serum creatinine levels above the upper limit of normal for their age should not receive AVANDAMET. In patients with advanced age, AVANDAMET should be carefully titrated to establish the minimum dose for adequate glycemic effect, because aging is associated with reduced renal function. [See Dosage and Administration (2.4) and Use in Specific Populations (8.5).]
Before initiation of therapy with AVANDAMET and at least annually thereafter, renal function should be assessed and verified as normal. In patients in whom development of renal dysfunction is anticipated, renal function should be assessed more frequently and AVANDAMET discontinued if evidence of renal impairment is present.
Medications That Affect Renal Function: Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or may interfere with the disposition of metformin, such as cationic drugs that are eliminated by renal tubular secretion [see Drug Interactions (7.2) and Clinical Pharmacology (12.4)], should be used with caution.
Hypoxic States: Cardiovascular collapse (shock) from whatever cause, acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients receiving AVANDAMET, the drug should be promptly discontinued.
Radiologic Studies With Intravascular Iodinated Contrast Materials: Intravascular contrast studies with iodinated materials can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin [see Contraindications (4)]. Therefore, in patients in whom any such study is planned, AVANDAMET should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal.
Surgical Procedures: Use of AVANDAMET should be temporarily suspended for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient's oral intake has resumed and renal function has been evaluated as normal.
Alcohol Intake: Alcohol potentiates the effect of metformin on lactate metabolism. Patients, therefore, should be warned against excessive alcohol intake, acute or chronic, while receiving AVANDAMET.
Change in Clinical Status of Patients With Previously Controlled Diabetes: A patient with type 2 diabetes previously well-controlled on AVANDAMET who develops laboratory abnormalities or clinical illness (especially vague and poorly defined illness) should be evaluated promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should include serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate, pyruvate, and metformin levels. If acidosis of either form occurs, AVANDAMET must be stopped immediately and other appropriate corrective measures initiated.
[See also Warnings and Precautions (5.6).]
5.2 Cardiac Failure
Rosiglitazone, like other thiazolidinediones, alone or in combination with other antidiabetic agents, can cause fluid retention, which may exacerbate or lead to heart failure. Patients should be observed for signs and symptoms of heart failure. If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of rosiglitazone must be considered [see Boxed Warning].
Patients with congestive heart failure (CHF) NYHA Class I and II treated with rosiglitazone have an increased risk of cardiovascular events. A 52-week, double-blind, placebo-controlled echocardiographic trial was conducted in 224 patients with type 2 diabetes mellitus and NYHA Class I or II CHF (ejection fraction ≤45%) on background antidiabetic and CHF therapy. An independent committee conducted a blinded evaluation of fluid-related events (including congestive heart failure) and cardiovascular hospitalizations according to predefined criteria (adjudication). Separate from the adjudication, other cardiovascular adverse events were reported by investigators. Although no treatment difference in change from baseline of ejection fractions was observed, more cardiovascular adverse events were observed with rosiglitazone treatment compared to placebo during the 52-week trial. (See Table 2.)
Table 2. Emergent Cardiovascular Adverse Events in Patients With Congestive Heart Failure (NYHA Class I and II) Treated With Rosiglitazone or Placebo (in Addition to Background Antidiabetic and CHF Therapy) Events Rosiglitazone Placebo N = 110
n (%)N = 114
n (%)Adjudicated Cardiovascular deaths 5 (5%) 4 (4%) CHF worsening 7 (6%) 4 (4%) – with overnight hospitalization 5 (5%) 4 (4%) – without overnight hospitalization 2 (2%) 0 (0%) New or worsening edema 28 (25%) 10 (9%) New or worsening dyspnea 29 (26%) 19 (17%) Increases in CHF medication 36 (33%) 20 (18%) Cardiovascular hospitalizationa 21 (19%) 15 (13%) Investigator-reported, non-adjudicated Ischemic adverse events 10 (9%) 5 (4%) – Myocardial infarction 5 (5%) 2 (2%) – Angina 6 (5%) 3 (3%) aIncludes hospitalization for any cardiovascular reason.
Initiation of AVANDAMET in patients with established NYHA Class III or IV heart failure is contraindicated. AVANDAMET is not recommended in patients with symptomatic heart failure. [See Boxed Warning.]
Patients experiencing acute coronary syndromes have not been studied in controlled clinical trials. In view of the potential for development of heart failure in patients having an acute coronary event, initiation of AVANDAMET is not recommended for patients experiencing an acute coronary event, and discontinuation of AVANDAMET during this acute phase should be considered.
Patients with NYHA Class III and IV cardiac status (with or without CHF) have not been studied in controlled clinical trials. AVANDAMET is not recommended in patients with NYHA Class III and IV cardiac status.
Congestive Heart Failure During Coadministration of Rosiglitazone With Insulin:In trials in which rosiglitazone was added to insulin, rosiglitazone increased the risk of congestive heart failure. Coadministration of rosiglitazone and insulin is not recommended. [See Indications and Usage (1) and Warnings and Precautions (5.3).]
In 7 controlled, randomized, double-blind trials which had durations from 16 to 26 weeks and which were included in a meta-analysis1[seeWarnings and Precautions (5.3)], patients with type 2 diabetes mellitus were randomized to coadministration of rosiglitazone and insulin (N = 1,018) or insulin (N = 815). In these 7 trials, rosiglitazone was added to insulin. These trials included patients with long-standing diabetes (median duration of 12 years) and a high prevalence of pre-existing medical conditions, including peripheral neuropathy, retinopathy, ischemic heart disease, vascular disease, and congestive heart failure. The total number of patients with emergent congestive heart failure was 23 (2.3%) and 8 (1.0%) in the rosiglitazone plus insulin and insulin groups, respectively.
Heart Failure in Observational Studies of Elderly Diabetic Patients Comparing Rosiglitazone to Pioglitazone: Three observational studies2-4 in elderly diabetic patients (age 65 years and older) found that rosiglitazone statistically significantly increased the risk of hospitalized heart failure compared to use of pioglitazone. One other observational study5 in patients with a mean age of 54 years, which also included an analysis in a subpopulation of patients >65 years of age, found no statistically significant increase in emergency department visits or hospitalization for heart failure in patients treated with rosiglitazone compared to pioglitazone in the older subgroup.
5.3 Major Adverse Cardiovascular Events
Cardiovascular adverse events have been evaluated in a meta-analysis of 52 clinical trials, in long-term, prospective, randomized, controlled trials, and in observational studies.
Meta-Analysis of Major Adverse Cardiovascular Events in a Group of 52 Clinical Trials: A meta-analysis was conducted retrospectively to assess cardiovascular adverse events reported across 52 double-blind, randomized, controlled clinical trials (mean duration 6 months).1 These trials had been conducted to assess glucose-lowering efficacy in type 2 diabetes. Prospectively planned adjudication of cardiovascular events did not occur in most of the trials. Some trials were placebo-controlled and some used active oral antidiabetic drugs as controls. Placebo-controlled trials included monotherapy trials (monotherapy with rosiglitazone versus placebo monotherapy) and add-on trials (rosiglitazone or placebo, added to sulfonylurea, metformin, or insulin). Active control trials included monotherapy trials (monotherapy with rosiglitazone versus sulfonylurea or metformin monotherapy) and add-on trials (rosiglitazone plus sulfonylurea or rosiglitazone plus metformin, versus sulfonylurea plus metformin). A total of 16,995 patients were included (10,039 in treatment groups containing rosiglitazone, 6,956 in comparator groups), with 5,167 patient-years of exposure to rosiglitazone and 3,637 patient-years of exposure to comparator. Cardiovascular events occurred more frequently for patients who received rosiglitazone than for patients who received comparators (see Table 3).
Table 3. Occurrence of Cardiovascular Events in a Meta-Analysis of 52 Clinical Trials Eventa Rosiglitazone
(N=10,039)
n (%)Comparator
(N=6,956)
n (%)MACE (a composite of myocardial infarction, cardiovascular death, or stroke) 70 (0.7) 39 (0.6) Myocardial Infarction 45 (0.4) 20 (0.3) Cardiovascular Death 17 (0.2) 9 (0.1) Stroke 18 (0.2) 16 (0.2) All-cause Death 29 (0.3) 17 (0.2) a Events are not exclusive: i.e., a patient with a cardiovascular death due to a myocardial infarction would be counted in 4 event categories (myocardial infarction; myocardial infarction, cardiovascular death, or stroke; cardiovascular death; all-cause death).
In this analysis, a statistically significant increased risk of myocardial infarction with rosiglitazone versus pooled comparators was observed. Analyses were performed using a composite of major adverse cardiovascular events (myocardial infarction, stroke, and cardiovascular death), referred to hereafter as MACE. Rosiglitazone had a statistically non-significant increased risk of MACE compared to the pooled comparators. A statistically significant increased risk of myocardial infarction and statistically non-significant increased risk of MACE with rosiglitazone was observed in the placebo-controlled trials. In the active-controlled trials, there was no increased risk of myocardial infarction or MACE. (See Figure 1 and Table 4.)
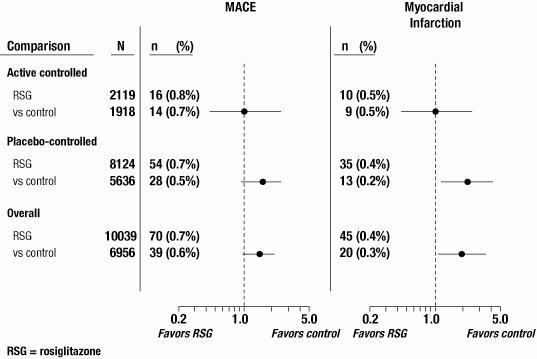
Figure 1. Forest Plot of Odds Ratios (95% Confidence Intervals) for MACE and Myocardial Infarction in the Meta-Analysis of 52 Clinical Trials
Table 4. Occurrence of MACE and Myocardial Infarction in a Meta-Analysis of 52 Clinical Trials by Trial Type MACE Myocardial Infarction N n (%) OR
(95%CI)n (%) OR
(95%CI)Active-
Controlled TrialsRSG 2,119 16 (0.8%) 1.05 10 (0.5%) 1.00 Control 1,918 14 (0.7%) (0.48, 2.34) 9 (0.5%) (0.36, 2.82) Placebo-
Controlled TrialsRSG 8,124 54 (0.7%) 1.53 35 (0.4%) 2.23 Placebo 5,636 28 (0.5%) (0.94, 2.54) 13 (0.2%) (1.14, 4.64)
OverallRSG 10,039 70 (0.7%) 1.44 45 (0.4%) 1.8 Control 6,956 39 (0.6%) (0.95, 2.20) 20 (0.3%) (1.03, 3.25) RSG = rosiglitazone
Of the placebo-controlled trials in the meta-analysis, 7 trials had patients randomized to rosiglitazone plus insulin or insulin. There were more patients in the rosiglitazone plus insulin group compared to the insulin group with myocardial infarctions, MACE, cardiovascular deaths, and all-cause deaths (see Table 5). The total number of patients with stroke was 5 (0.5%) and 4 (0.5%) in the rosiglitazone plus insulin and insulin groups, respectively. The use of rosiglitazone in combination with insulin may increase the risk of myocardial infarction [See Warnings and Precautions (5.1).]
Table 5. Occurrence of Cardiovascular Events for Rosiglitazone in Combination With Insulin in a Meta-Analysis of 52 Clinical Trials
EventaRosiglitazone
(N=1,018)
(%)Insulin
(N = 815)
(%)
OR (95% CI)MACE (a composite of myocardial infarction, cardiovascular death, or stroke) 1.3 0.6 2.14 (0.70, 7.83) Myocardial infarction 0.6 0.1 5.6 (0.67, 262.7) Cardiovascular death 0.4 0.0 ND, (0.47, ∞) All cause death 0.6 0.2 2.19 (0.38, 22.61) ND = not defined
a Events are not exclusive: i.e., a patient with a cardiovascular death due to a myocardial infarction would be counted in 4 event categories (myocardial infarction; myocardial infarction, cardiovascular death, or stroke; cardiovascular death; all-cause death).
Myocardial Infarction Events in Large, Long-Term, Prospective, Randomized, Controlled Trials of Rosiglitazone: Data from 3 large, long-term, prospective, randomized, controlled clinical trials of rosiglitazone were assessed separately from the meta-analysis.6-8 These 3 trials included a total of 14,067 patients (treatment groups containing rosiglitazone N = 6,311; comparator groups N = 7,756), with patient-year exposure of 24,534 patient-years for rosiglitazone and 28,882 patient-years for comparator. Patient populations in the trials included patients with impaired glucose tolerance, patients with type 2 diabetes who were initiating oral agent monotherapy, and patients with type 2 diabetes who had failed monotherapy and were initiating dual oral agent therapy. Duration of follow-up exceeded 3 years in each trial.
In each of these trials, there was a statistically non-significant increase in the risk of myocardial infarction for rosiglitazone versus comparator medications.
In a long-term, randomized, placebo-controlled, 2x2 factorial trial intended to evaluate rosiglitazone, and separately ramipril (an angiotensin converting enzyme inhibitor [ACEI]), on progression to overt diabetes in 5,269 subjects with glucose intolerance, the incidence of myocardial infarction was higher in the subset of subjects who received rosiglitazone in combination with ramipril than among subjects who received ramipril alone but not in the subset of subjects who received rosiglitazone alone compared to placebo.6 The higher incidence of myocardial infarction among subjects who received rosiglitazone in combination with ramipril was not confirmed in the two other large (total N = 8,798) long-term, randomized, active-controlled clinical trials conducted in patients with type 2 diabetes, in which 30% and 40% of patients in the two trials reported angiotensin-converting enzyme inhibitor use at baseline.7,8
There have been no adequately designed clinical trials directly comparing rosiglitazone to pioglitazone on cardiovascular risks. However, in a long-term, randomized, placebo-controlled cardiovascular outcomes trial comparing pioglitazone to placebo in patients with type 2 diabetes mellitus and prior macrovascular disease, pioglitazone was not associated with an increased risk of myocardial infarction or total mortality.9
The increased risk of myocardial infarction observed in the meta-analysis and large, long-term controlled clinical trials, and the increased risk of MACE observed in the meta-analysis described above, have not translated into a consistent finding of excess mortality from controlled clinical trials or observational studies. Clinical trials have not shown any difference between rosiglitazone and comparator medications in overall mortality or CV-related mortality.
Mortality in Observational Studies of Rosiglitazone Compared to Pioglitazone: Three observational studies in elderly diabetic patients (age 65 years and older) found that rosiglitazone statistically significantly increased the risk of all-cause mortality compared to use of pioglitazone.2-4 One observational study5 in patients with a mean age of 54 years found no difference in all-cause mortality between patients treated with rosiglitazone compared to pioglitazone and reported similar results in the subpopulation of patients >65 years of age. One additional small, prospective, observational study10 found no statistically significant differences for CV mortality and all-cause mortality in patients treated with rosiglitazone compared to pioglitazone.
5.4 Edema
AVANDAMET should be used with caution in patients with edema. In a clinical trial in healthy volunteers who received rosiglitazone 8 mg once daily for 8 weeks, there was a statistically significant increase in median plasma volume compared to placebo. Since thiazolidinediones, including rosiglitazone, can cause fluid retention, which can exacerbate or lead to congestive heart failure, AVANDAMET should be used with caution in patients at risk for heart failure. Patients should be monitored for signs and symptoms of heart failure [see Boxed Warning, Warnings and Precautions (5.2), and Patient Counseling Information (17.1)].
In controlled clinical trials of patients with type 2 diabetes, mild to moderate edema was reported in patients treated with rosiglitazone, and may be dose-related. Patients with ongoing edema were more likely to have adverse events associated with edema if started on combination therapy with insulin and rosiglitazone [see Adverse Reactions (6.1)]. The use of AVANDAMET in combination with insulin is not recommended. [See Warnings and Precautions (5.2, 5.3).]
5.5 Weight Gain
Dose-related weight gain was seen with rosiglitazone alone and rosiglitazone together with other hypoglycemic agents (see Table 6). No overall change in median weight was observed with AVANDAMET in drug-naïve patients. The mechanism of weight gain with rosiglitazone is unclear but probably involves a combination of fluid retention and fat accumulation.
Table 6. Weight Changes (kg) From Baseline at Endpoint During Clinical Trials [Median (25th, 75th, Percentile)] Monotherapy Duration Control Group Rosiglitazone 4 mg Rosiglitazone 8 mg 26 weeks Placebo -0.9 (-2.8, 0.9)
N = 2101.0 (0.9, 3.6)
N = 4363.1 (1.1, 5.8)
N = 43952 weeks Sulfonylurea 2.0 (0, 4.0)
N = 1732.0 (-0.6, 4.0)
N = 1502.6 (0, 5.3)
N = 157Combination Therapy Rosiglitazone + Control Therapy Duration Control Group Rosiglitazone 4 mg Rosiglitazone 8 mg 24-26 weeks Sulfonylurea 0 (-1.0, 1.3)
N = 1,1552.2 (0.5, 4.0)
N = 6133.5 (1.4, 5.9)
N = 84126 weeks Metformin -1.4 (-3.2, 0.2)
N = 1750.8 (-1.0, 2.6)
N = 1002.1 (0, 4.3)
N = 18426 weeks Insulin 0.9 (-0.5, 2.7)
N = 1624.1 (1.4, 6.3)
N = 1645.4 (3.4, 7.3)
N = 150AVANDAMET + Insulin Duration Control Group AVANDAMET + Insulin 24 weeks Insulin 2.6 kg (0.3, 4.8)
N = 1453.3 kg (1.5, 6.0)
N = 147In a 4- to 6-year, monotherapy, comparative trial (ADOPT) in patients recently diagnosed with type 2 diabetes not previously treated with antidiabetic medication, the median weight change (25th, 75th percentiles) from baseline at 4 years was 3.5 kg (0.0, 8.1) for rosiglitazone, 2.0 kg (-1.0, 4.8) for glyburide, and -2.4 kg (-5.4, 0.5) for metformin.
In postmarketing experience with rosiglitazone alone or in combination with other hypoglycemic agents, there have been rare reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure [see Boxed Warning].
5.6 Hepatic Effects
Metformin: Since impaired hepatic function has been associated with some cases of lactic acidosis, AVANDAMET should generally be avoided in patients with clinical or laboratory evidence of hepatic disease.
Rosiglitazone: Liver enzymes should be measured prior to the initiation of therapy with AVANDAMET in all patients and periodically thereafter per the clinical judgment of the healthcare professional. Therapy with AVANDAMET should not be initiated in patients with increased baseline liver enzyme levels (ALT >2.5X upper limit of normal). Patients with mildly elevated liver enzymes (ALT levels ≤2.5X upper limit of normal) at baseline or during therapy with AVANDAMET should be evaluated to determine the cause of the liver enzyme elevation. Initiation of, or continuation of, therapy with AVANDAMET in patients with mild liver enzyme elevations should proceed with caution and include close clinical follow-up, including more frequent liver enzyme monitoring, to determine if the liver enzyme elevations resolve or worsen. If at any time ALT levels increase to >3X the upper limit of normal in patients on therapy with AVANDAMET, liver enzyme levels should be rechecked as soon as possible. If ALT levels remain >3X the upper limit of normal, therapy with AVANDAMET should be discontinued.
If any patient develops symptoms suggesting hepatic dysfunction, which may include unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, and/or dark urine, liver enzymes should be checked. The decision whether to continue the patient on therapy with AVANDAMET should be guided by clinical judgment pending laboratory evaluations. If jaundice is observed, drug therapy should be discontinued.
In addition, if the presence of hepatic disease or hepatic dysfunction of sufficient magnitude to predispose to lactic acidosis is confirmed, therapy with AVANDAMET should be discontinued.
5.7 Macular Edema
Macular edema has been reported in postmarketing experience in some diabetic patients who were taking rosiglitazone or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but some patients appear to have been diagnosed on routine ophthalmologic examination. Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of their thiazolidinedione. Patients with diabetes should have regular eye exams by an ophthalmologist, per the Standards of Care of the American Diabetes Association. Additionally, any diabetic who reports any kind of visual symptom should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings. [See Adverse Reactions (6.3).]
5.8 Fractures
In a 4- to 6-year comparative trial (ADOPT) of glycemic control with monotherapy in drug-naïve patients recently diagnosed with type 2 diabetes mellitus, an increased incidence of bone fracture was noted in female patients taking rosiglitazone. Over the 4- to 6-year period, the incidence of bone fracture in females was 9.3% (60/645) for rosiglitazone versus 3.5% (21/605) for glyburide and 5.1% (30/590) for metformin. This increased incidence was noted after the first year of treatment and persisted during the course of the trial. The majority of the fractures in the women who received rosiglitazone occurred in the upper arm, hand, and foot. These sites of fracture are different from those usually associated with postmenopausal osteoporosis (e.g., hip or spine). Other trials suggest that this risk may also apply to men, although the risk of fracture among women appears higher than that among men. The risk of fracture should be considered in the care of patients treated with rosiglitazone, and attention given to assessing and maintaining bone health according to current standards of care.
5.9 Hematologic Effects
Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with rosiglitazone [see Adverse Reactions (6.2)]. The observed changes may be related to the increased plasma volume observed with treatment with rosiglitazone and may be dose-related. The decrease in hemoglobin was seen more frequently in combination rosiglitazone and metformin therapy than in rosiglitazone therapy alone. Vitamin B12 deficiency may contribute to the observed reductions in hemoglobin [see Warnings and Precautions (5.10)]. Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red blood cell indices) should be performed, at least on an annual basis.
5.10 Vitamin B12 Levels
In controlled clinical trials of metformin of 29 weeks’ duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurements at 2- to 3-year intervals may be useful. Vitamin B12 deficiency should be excluded if megaloblastic anemia is suspected. [See Warnings and Precautions (5.9).]
5.11 Diabetes and Blood Glucose Control
Periodic fasting blood glucose and HbA1c measurements should be performed to monitor therapeutic response.
When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of glycemic control may occur. At such times, it may be necessary to withhold AVANDAMET and temporarily administer insulin. AVANDAMET may be reinstituted after the acute episode is resolved.
Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with hypoglycemic agents (such as sulfonylureas or insulin) or ethanol. Elderly, debilitated or malnourished patients, and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly and in people who are taking β-adrenergic blocking drugs.
Patients receiving rosiglitazone in combination with other hypoglycemic agents may be at risk for hypoglycemia, and a reduction in the dose of the concomitant agent may be necessary.
5.12 Ovulation
Therapy with rosiglitazone, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking AVANDAMET [see Use in Specific Populations (8.1)]. Thus, adequate contraception in premenopausal women should be recommended. This possible effect has not been specifically investigated in clinical trials; therefore, the frequency of this occurrence is not known.
Although hormonal imbalance has been seen in preclinical studies [see Nonclinical Toxicology (13.1)], the clinical significance of this finding is not known. If unexpected menstrual dysfunction occurs, the benefits of continued therapy with AVANDAMET should be reviewed.
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The incidence and types of adverse events reported in controlled, 26-week clinical trials of rosiglitazone administered in combination with metformin 2,500 mg/day in comparison to adverse reactions reported in association with rosiglitazone and metformin monotherapies are shown in Table 7. Overall, the types of adverse reactions without regard to causality reported when rosiglitazone was used in combination with metformin were similar to those reported during monotherapy with rosiglitazone.
Table 7. Adverse Events (≥5% for Rosiglitazone Plus Metformin) Reported by Patients in 26-week Double-blind Clinical Trials of Rosiglitazone Added to Metformin Therapy Rosiglitazone + Metformin Rosiglitazone Placebo Metformin N = 338 N = 2,526 N = 601 N = 225 Preferred term % % % % Upper respiratory tract infection 16.0 9.9 8.7 8.9 Diarrhea 12.7 2.3 3.3 15.6 Injury 8.0 7.6 4.3 7.6 Anemia 7.1 1.9 0.7 2.2 Headache 6.5 5.9 5.0 8.9 Sinusitis 6.2 3.2 4.5 5.3 Fatigue 5.9 3.6 5.0 4.0 Back pain 5.0 4.0 3.8 4.0 Viral infection 5.0 3.2 4.0 3.6 Arthralgia 5.0 3.0 4.0 2.2 Reports of hypoglycemia in patients treated with rosiglitazone added to maximum metformin therapy in double-blind trials were more frequent (3.0%) than in patients treated with rosiglitazone (0.6%) or metformin monotherapies (1.3%) or placebo (0.2%). Overall, anemia and edema were generally mild to moderate in severity and usually did not require discontinuation of treatment with rosiglitazone.
Edema was reported in 4.8% of patients receiving rosiglitazone compared to 1.3% on placebo, and 2.2% on metformin monotherapy and 4.4% on rosiglitazone in combination with maximum doses of metformin.
Reports of anemia (7.1%) were greater in patients treated with rosiglitazone added to metformin compared to monotherapy with rosiglitazone. Lower pre-treatment hemoglobin/hematocrit levels in patients enrolled in the metformin and rosiglitazone combination therapy clinical trials may have contributed to the higher reporting rate of anemia in these trials [see Adverse Reactions (6.2)].
Combination with Insulin: The incidence of hypoglycemia (confirmed by fingerstick blood glucose concentration ≤50 mg/dL) was 14% for patients on AVANDAMET plus insulin compared to 10% for patients on insulin monotherapy.
The incidence of edema was 7% when insulin was added to AVANDAMET compared to 3% with insulin monotherapy. This trial excluded patients with pre-existing heart failure or new or worsening edema on AVANDAMET therapy. However, in 26-week double-blind, fixed-dose trials of rosiglitazone added to insulin, edema was reported with higher frequency (rosiglitazone in combination with insulin, 14.7%; insulin, 5.4%) [see Warnings and Precautions (5.2)].
In trials in which rosiglitazone was added to insulin, rosiglitazone increased the risk of congestive heart failure. The use of rosiglitazone in combination with insulin may increase the risk of myocardial infarction [see Warnings and Precautions (5.2, 5.3)].
In a trial in which insulin was added to AVANDAMET, no myocardial ischemia was observed in the insulin group (N = 158), and no congestive heart failure was reported in either group. There was one myocardial ischemic event and one sudden death in the group receiving AVANDAMET plus insulin (N = 161). [See Warnings and Precautions (5.2).]
The incidence of anemia was 2% for AVANDAMET in combination with insulin compared to 1% for insulin monotherapy.
A long-term, 4- to 6-year trial (ADOPT) compared the use of rosiglitazone (n = 1,456), glyburide (n = 1,441), and metformin (n = 1,454) as monotherapy in patients recently diagnosed with type 2 diabetes who were not previously treated with antidiabetic medication. Table 8 presents adverse reactions without regard to causality; rates are expressed per 100 patient-years (PY) exposure to account for the differences in exposure to trial medication across the 3 treatment groups.
In ADOPT, fractures were reported in a greater number of women treated with rosiglitazone (9.3%, 2.7/100 patient-years) compared to glyburide (3.5%, 1.3/100 patient-years) or metformin (5.1%, 1.5/100 patient-years). The majority of the fractures in the women who received rosiglitazone were reported in the upper arm, hand, and foot. [See Warnings and Precautions (5.8).] The observed incidence of fractures for male patients was similar among the 3 treatment groups.
Table 8. On-Therapy Adverse Events (≥5 Events/100 Patient-Years [PY]) in Any Treatment Group Reported in a 4- to 6-Year Clinical Trial of Rosiglitazone as Monotherapy (ADOPT) Rosiglitazone Glyburide Metformin N = 1,456 N = 1,441 N = 1,454 PY = 4,954 PY = 4,244 PY = 4,906 Nasopharyngitis 6.3 6.9 6.6 Back pain 5.1 4.9 5.3 Arthralgia 5.0 4.8 4.2 Hypertension 4.4 6.0 6.1 Upper respiratory tract infection 4.3 5.0 4.7 Hypoglycemia 2.9 13.0 3.4 Diarrhea 2.5 3.2 6.8 6.2 Laboratory Abnormalities
Hematologic: Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with rosiglitazone (mean decreases in individual trials as much as 1.0 gram/dL hemoglobin and as much as 3.3% hematocrit). The changes occurred primarily during the first 3 months following initiation of rosiglitazone therapy or following an increase in rosiglitazone dose. The time course and magnitude of decreases were similar in patients treated with a combination of rosiglitazone and other hypoglycemic agents or monotherapy with rosiglitazone. Pre-treatment levels of hemoglobin and hematocrit were lower in patients in metformin combination trials and may have contributed to the higher reporting rate of anemia. In a single trial in pediatric patients, decreases in hemoglobin and hematocrit (mean decreases of 0.29 g/dL and 0.95%, respectively) were reported with rosiglitazone. White blood cell counts also decreased slightly in adult patients treated with rosiglitazone. Decreases in hematologic parameters may be related to increased plasma volume observed with rosiglitazone treatment.
In controlled clinical trials of metformin of 29 weeks’ duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such a decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation.
Lipids: Changes in serum lipids have been observed following treatment with rosiglitazone in adults [see Clinical Pharmacology (12.2)].
Serum Transaminase Levels: In pre-approval clinical trials in 4,598 patients treated with rosiglitazone encompassing approximately 3,600 patient years of exposure, and in a long-term 4- to 6-year trial in 1,456 patients treated with rosiglitazone (4,954 patient-years exposure), there was no evidence of drug-induced hepatotoxicity.
In pre-approval controlled trials, 0.2% of patients treated with rosiglitazone had reversible elevations in ALT >3X the upper limit of normal compared to 0.2% on placebo and 0.5% on active comparators. The ALT elevations in patients treated with rosiglitazone were reversible. Hyperbilirubinemia was found in 0.3% of patients treated with rosiglitazone compared with 0.9% treated with placebo and 1% in patients treated with active comparators. In pre-approval clinical trials, there were no cases of idiosyncratic drug reactions leading to hepatic failure. [See Warnings and Precautions (5.6).]
In the 4- to 6-year ADOPT trial, patients treated with rosiglitazone (4,954 patient-years exposure), glyburide (4,244 patient-years exposure) or metformin (4,906 patient-years exposure) as monotherapy, had the same rate of ALT increase to >3X upper limit of normal (0.3 per 100 patient-years exposure).
6.3 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the events described below have been identified during post-approval use of AVANDAMET or its individual components. Because these events are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or to always establish a causal relationship to drug exposure.
In patients receiving thiazolidinedione therapy, serious adverse events with or without a fatal outcome, potentially related to volume expansion (e.g., congestive heart failure, pulmonary edema, and pleural effusions) have been reported [see Boxed Warning and Warnings and Precautions (5.2)].
There are postmarketing reports with rosiglitazone of hepatitis, hepatic enzyme elevations to 3 or more times the upper limit of normal, and hepatic failure with and without fatal outcome, although causality has not been established.
There are postmarketing reports with rosiglitazone of rash, pruritus, urticaria, angioedema, anaphylactic reaction, Stevens-Johnson syndrome, and new onset or worsening diabetic macular edema with decreased visual acuity [see Warnings and Precautions (5.7)].
(See also GLUCOPHAGE® prescribing information.)
-
7 DRUG INTERACTIONS
7.1 Drugs Metabolized by Cytochrome P450
An inhibitor of CYP2C8 (e.g., gemfibrozil) may increase the AUC of rosiglitazone and an inducer of CYP2C8 (e.g., rifampin) may decrease the AUC of rosiglitazone. Therefore, if an inhibitor or an inducer of CYP2C8 is started or stopped during treatment with rosiglitazone, changes in diabetes treatment may be needed based upon clinical response. [See Clinical Pharmacology (12.4).]
7.2 Cationic Drugs
Although drug interactions for metformin with cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, and vancomycin) remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of AVANDAMET and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system. [See Warnings and Precautions (5.1) and Clinical Pharmacology (12.4).]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
All pregnancies have a background risk of birth defects, loss, or other adverse outcome regardless of drug exposure. This background risk is increased in pregnancies complicated by hyperglycemia and may be decreased with good metabolic control. It is essential for patients with diabetes or history of gestational diabetes to maintain good metabolic control before conception and throughout pregnancy. Careful monitoring of glucose control is essential in such patients. Most experts recommend that insulin monotherapy be used during pregnancy to maintain blood glucose levels as close to normal as possible. AVANDAMET should not be used during pregnancy.
Human Data: There are no adequate and well-controlled trials with AVANDAMET or its individual components in pregnant women. Rosiglitazone has been reported to cross the human placenta and be detectable in fetal tissue. The clinical significance of these findings is unknown.
Animal Studies: No animal studies have been conducted with AVANDAMET. The following data are based on findings in studies performed with rosiglitazone or metformin individually.
Rosiglitazone: There was no effect on implantation or the embryo with rosiglitazone treatment during early pregnancy in rats, but treatment during mid-late gestation was associated with fetal death and growth retardation in both rats and rabbits. Teratogenicity was not observed at doses up to 3 mg/kg in rats and 100 mg/kg in rabbits (approximately 20 and 75 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively). Rosiglitazone caused placental pathology in rats (3 mg/kg/day). Treatment of rats during gestation through lactation reduced litter size, neonatal viability, and postnatal growth, with growth retardation reversible after puberty. For effects on the placenta, embryo/fetus, and offspring, the no-effect dose was 0.2 mg/kg/day in rats and 15 mg/kg/day in rabbits. These no-effect levels are approximately 4 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET. Rosiglitazone reduced the number of uterine implantations and live offspring when juvenile female rats were treated at 40 mg/kg/day from 27 days of age through to sexual maturity (approximately 68 times human AUC at the maximum recommended daily dose). The no-effect level was 2 mg/kg/day (approximately 4 times human AUC at the maximum recommended daily dose). There was no effect on pre- or post-natal survival or growth.
Metformin: Metformin was not teratogenic in rats and rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 2 and 6 times the maximum recommended human daily dose of 2,000 mg based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
8.2 Labor and Delivery
The effect of AVANDAMET or its components on labor and delivery in humans is unknown.
8.3 Nursing Mothers
No studies have been conducted with AVANDAMET. In studies performed with the individual components, both rosiglitazone-related material and metformin were detectable in milk from lactating rats. It is not known whether rosiglitazone or metformin is excreted in human milk. Because many drugs are excreted in human milk, AVANDAMET should not be administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of AVANDAMET in pediatric patients have not been established. AVANDAMET and rosiglitazone are not indicated for use in pediatric patients.
8.5 Geriatric Use
Metformin is known to be substantially excreted by the kidney and because the risk of serious adverse reactions to the drug is greater in patients with impaired renal function, AVANDAMET should only be used in patients with normal renal function [see Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)]. Because reduced renal function is associated with increasing age, AVANDAMET should be used with caution in elderly patients. Care should be taken in dose selection and should be based on careful and regular monitoring of renal function. Generally, elderly patients should not be titrated to the maximum dose of AVANDAMET [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
Rosiglitazone: Limited data are available with regard to overdosage in humans. In clinical trials in volunteers, rosiglitazone has been administered at single oral doses of up to 20 mg and was well tolerated. In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s clinical status.
Metformin: Hypoglycemia has not been seen with ingestion of up to 85 grams of metformin, although lactic acidosis has occurred in such circumstances [see Warnings and Precautions (5.1)]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated metformin from patients in whom metformin overdosage is suspected.
-
11 DESCRIPTION
AVANDAMET contains 2 oral antidiabetic drugs: rosiglitazone maleate and metformin hydrochloride.
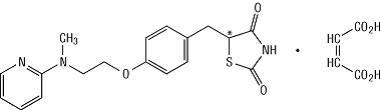
Rosiglitazone maleate is an oral antidiabetic agent, which acts primarily by increasing insulin sensitivity. Rosiglitazone improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally related to the sulfonylureas, the biguanides, or the alpha-glucosidase inhibitors. Chemically, rosiglitazone maleate is (±)-5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, (Z)-2-butenedioate (1:1) with a molecular weight of 473.52 (357.44 free base). The molecule has a single chiral center and is present as a racemate. Due to rapid interconversion, the enantiomers are functionally indistinguishable. The molecular formula is C18H19N3O3SC4H4O4. Rosiglitazone maleate is a white to off-white solid with a melting point range of 122° to 123°C. The pKa values of rosiglitazone maleate are 6.8 and 6.1. It is readily soluble in ethanol and a buffered aqueous solution with pH of 2.3; solubility decreases with increasing pH in the physiological range. The structural formula of rosiglitazone maleate is:
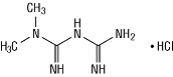
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antidiabetic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula of metformin hydrochloride is:
AVANDAMET is available for oral administration as film-coated tablets containing rosiglitazone maleate and metformin hydrochloride equivalent to: 2 mg rosiglitazone with 500 mg metformin hydrochloride (2 mg/500 mg), 4 mg rosiglitazone with 500 mg metformin hydrochloride (4 mg/500 mg), 2 mg rosiglitazone with 1,000 mg metformin hydrochloride (2 mg/1,000 mg), and 4 mg rosiglitazone with 1,000 mg metformin hydrochloride (4 mg/1,000 mg). Inactive ingredients are: Hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone 29-32, sodium starch glycolate, titanium dioxide, and 1 or more of the following: Red and yellow iron oxides.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
AVANDAMET: AVANDAMET combines 2 antidiabetic agents with different mechanisms of action to improve glycemic control in patients with type 2 diabetes: Rosiglitazone, a member of the thiazolidinedione class, and metformin, a member of the biguanide class. Thiazolidinediones are insulin sensitizing agents that act primarily by enhancing peripheral glucose utilization, whereas biguanides act primarily by decreasing endogenous hepatic glucose production.
Rosiglitazone: Rosiglitazone improves glycemic control by improving insulin sensitivity. Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator–activated receptor-gamma (PPARγ). In humans, PPAR receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARγ-responsive genes also participate in the regulation of fatty acid metabolism.
Insulin resistance is a common feature characterizing the pathogenesis of type 2 diabetes. The antidiabetic activity of rosiglitazone has been demonstrated in animal models of type 2 diabetes in which hyperglycemia and/or impaired glucose tolerance is a consequence of insulin resistance in target tissues. Rosiglitazone reduces blood glucose concentrations and reduces hyperinsulinemia in the ob/ob obese mouse, db/db diabetic mouse, and fa/fa fatty Zucker rat.
In animal models, the antidiabetic activity of rosiglitazone was shown to be mediated by increased sensitivity to insulin's action in the liver, muscle, and adipose tissue. Pharmacologic studies in animal models indicate that rosiglitazone improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. The expression of the insulin-regulated glucose transporter GLUT-4 was increased in adipose tissue. Rosiglitazone did not induce hypoglycemia in animal models of type 2 diabetes and/or impaired glucose tolerance.
Metformin: Metformin is an antidiabetic agent, which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antidiabetic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and increases peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in either patients with type 2 diabetes or normal subjects except in special circumstances [see Warnings and Precautions (5.11)] and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
12.2 Pharmacodynamics
In all 26-week controlled trials, across the recommended dose range, rosiglitazone as monotherapy was associated with increases in total cholesterol, LDL-cholesterol and HDL-cholesterol and decreases in free fatty acids.
The lipid profiles of AVANDAMET as well as rosiglitazone and metformin monotherapies in patients who have inadequate glycemic control on diet and exercise are shown in Table 9.
Table 9. Summary of Meana Lipid Changes in a 32-Week Trial of AVANDAMET in Patients with Type 2 Diabetes Mellitus Who Have Inadequate Glycemic Control on Diet and Exercise AVANDAMET
Nb = 132Rosiglitazone
Nb = 128Metformin
Nb = 117Total Cholesterol (mg/dL) Baseline (mean) 200.4 198.4 201.6 % Change from baseline (mean) -2.2% 5.3% -9.0% LDL (mg/dL) Baseline (mean) 113.8 114.6 116.0 % Change from baseline (mean) -0.2% 4.5% -10.7% HDL (mg/dL) Baseline (mean) 42.6 42.8 42.9 % Change from baseline (mean) 5.8% 3.1% 0.0% Triglycerides (mg/dL) Baseline (mean) 180.3 166.6 175.7 % Change from baseline (mean) -18.7% -4.8% -15.4% aData presented as geometric means throughout table.
bN = number of subjects with a baseline and end of treatment value.
The pattern of LDL, HDL, and total cholesterol changes following therapy with rosiglitazone added to metformin was generally similar to those seen with rosiglitazone monotherapy, and a small decrease in mean triglycerides was observed with the combination therapy.
12.3 Pharmacokinetics
Absorption: AVANDAMET: In a bioequivalence and dose proportionality trial of AVANDAMET 4 mg/500 mg, both the rosiglitazone component and the metformin component were bioequivalent to coadministered 4 mg rosiglitazone tablet and 500 mg metformin tablet under fasted conditions (see Table 10). In this trial, dose proportionality of rosiglitazone in the combination formulations of 1 mg/500 mg and 4 mg/500 mg was demonstrated.
Table 10. Mean (SD) Pharmacokinetic Parameters for Rosiglitazone and Metformin Pharmacokinetic Parameter Regimen N AUC0-inf (ng.h/mL) Cmax (ng/mL) Tmaxa
(h)T½
(h)Rosiglitazone A 25 1,442
(324)242
(70)0.95
(0.48-2.47)4.26
(1.18)B 25 1,398
(340)254
(69)0.57
(0.43-2.58)3.95
(0.81)C 24 349
(91)63.0
(15.0)0.57
(0.47-1.45)3.87
(0.88)Metformin A 25 7,116
(2,096)1,106
(329)2.97
(1.02-4.02)3.46
(0.96)B 25 7,413
(1,838)1,135
(253)2.50
(1.03-3.98)3.36
(0.54)C 24 6,945
(2,045)1,080
(327)2.97
(1.00-5.98)3.35
(0.59)aMedian and range presented for Tmax.
Regimen A = 4 mg/500 mg AVANDAMET; Regimen B = 4 mg rosiglitazone tablet + 500 mg metformin tablet; Regimen C = 1 mg/500 mg AVANDAMET
Administration of AVANDAMET 4 mg/500 mg with food resulted in no change in overall exposure (AUC) for either rosiglitazone or metformin. However, there were decreases in Cmax of both components (22% for rosiglitazone and 15% for metformin, respectively) and a delay in Tmax of both components (1.5 hours for rosiglitazone and 0.5 hours for metformin, respectively). These changes are not likely to be clinically significant. The pharmacokinetics of both the rosiglitazone component and the metformin component of AVANDAMET when taken with food were similar to the pharmacokinetics of rosiglitazone and metformin when administered concomitantly as separate tablets with food.
Absorption: Rosiglitazone: The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Maximum plasma concentration (Cmax) and the area under the curve (AUC) of rosiglitazone increase in a dose-proportional manner over the therapeutic dose range.
Absorption: Metformin: The absolute bioavailability of a 500 mg metformin tablet given under fasting conditions is approximately 50% to 60%. Trials using single oral doses of metformin tablets of 500 mg to 1,500 mg, and 850 mg to 2,550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination.
Distribution: Rosiglitazone: The mean (CV%) oral volume of distribution (Vss/F) of rosiglitazone is approximately 17.6 (30%) liters, based on a population pharmacokinetic analysis. Rosiglitazone is approximately 99.8% bound to plasma proteins, primarily albumin.
Distribution: Metformin: The apparent volume of distribution (V/F) of metformin following single oral doses of 850 mg metformin averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin, steady-state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 mcg/mL. During controlled clinical trials, maximum metformin plasma levels did not exceed 5 mcg/mL, even at maximum doses.
Metabolism and Excretion: Rosiglitazone: Rosiglitazone is extensively metabolized with no unchanged drug excreted in the urine. The major routes of metabolism were N-demethylation and hydroxylation, followed by conjugation with sulfate and glucuronic acid. All the circulating metabolites are considerably less potent than parent and, therefore, are not expected to contribute to the insulin-sensitizing activity of rosiglitazone. In vitro data demonstrate that rosiglitazone is predominantly metabolized by Cytochrome P450 (CYP) isoenzyme 2C8, with CYP2C9 contributing as a minor pathway. Following oral or intravenous administration of [14C]rosiglitazone maleate, approximately 64% and 23% of the dose was eliminated in the urine and in the feces, respectively. The plasma half-life of [14C]related material ranged from 103 to 158 hours. The elimination half-life is 3 to 4 hours and is independent of dose.
Metabolism and Excretion: Metformin: Intravenous single-dose trials in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Renal clearance is approximately 3.5 times greater than creatinine clearance which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Special Populations: Renal Impairment: In subjects with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance [see Warnings and Precautions (5.1) and GLUCOPHAGE prescribing information]. Since metformin is contraindicated in patients with renal impairment, administration of AVANDAMET is contraindicated in these patients.
Hepatic Impairment: Unbound oral clearance of rosiglitazone was significantly lower in patients with moderate to severe liver disease (Child-Pugh Class B/C) compared to healthy subjects. As a result, unbound Cmax and AUC0-inf were increased 2- and 3-fold, respectively. Elimination half-life for rosiglitazone was about 2 hours longer in patients with liver disease, compared to healthy subjects.
Therapy with AVANDAMET should not be initiated if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5X upper limit of normal) at baseline [see Warnings and Precautions (5.6)].
No pharmacokinetic trials of metformin have been conducted in subjects with hepatic insufficiency.
Geriatric: Results of the population pharmacokinetics analysis (N = 716 <65 years; N = 331 ≥65 years) showed that age does not significantly affect the pharmacokinetics of rosiglitazone. However, limited data from controlled pharmacokinetic trials of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function [see Use in Specific Populations (8.5) and GLUCOPHAGE prescribing information]. Metformin treatment and therefore treatment with AVANDAMET should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced [see Dosage and Administration (2) and Warnings and Precautions (5.1)].
Gender: Results of the population pharmacokinetics analysis showed that the mean oral clearance of rosiglitazone in female patients (N = 405) was approximately 6% lower compared to male patients of the same body weight (N = 642). In rosiglitazone and metformin combination trials, efficacy was demonstrated with no gender differences in glycemic response.
Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes when analyzed according to gender (males = 19, females = 16). Similarly, in controlled clinical trials in patients with type 2 diabetes, the antihyperglycemic effect of metformin tablets was comparable in males and females.
Race: Results of a population pharmacokinetic analysis including subjects of white, black, and other ethnic origins indicate that race has no influence on the pharmacokinetics of rosiglitazone.
No trials of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical trials of metformin in patients with type 2 diabetes, the antihyperglycemic effect was comparable in whites (N = 249), blacks (N = 51), and Hispanics (N = 24).
Pediatric: No pharmacokinetic data from trials in pediatric subjects are available for AVANDAMET.
12.4 Drug-Drug Interactions
Rosiglitazone: Drugs That Inhibit, Induce, or are Metabolized by Cytochrome P450: In vitro drug metabolism studies suggest that rosiglitazone does not inhibit any of the major P450 enzymes at clinically relevant concentrations. In vitro data demonstrate that rosiglitazone is predominantly metabolized by CYP2C8, and to a lesser extent, 2C9. [See Drug Interactions (7.1).]
Rosiglitazone (4 mg twice daily) was shown to have no clinically relevant effect on the pharmacokinetics of nifedipine and oral contraceptives (ethinyl estradiol and norethindrone), which are predominantly metabolized by CYP3A4.
Gemfibrozil: Concomitant administration of gemfibrozil (600 mg twice daily), an inhibitor of CYP2C8, and rosiglitazone (4 mg once daily) for 7 days increased rosiglitazone AUC by 127%, compared to the administration of rosiglitazone (4 mg once daily) alone. Given the potential for dose-related adverse events with rosiglitazone, a decrease in the dose of rosiglitazone may be needed when gemfibrozil is introduced. [See Drug Interactions (7.1).]
Rifampin: Rifampin administration (600 mg once a day), an inducer of CYP2C8, for 6 days is reported to decrease rosiglitazone AUC by 66%, compared to the administration of rosiglitazone (8 mg) alone.11[See Drug Interactions (7.1).]
Metformin: Cationic Drugs: Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, and vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Such interaction between metformin and oral cimetidine has been observed in normal healthy volunteers in both single- and multiple-dose, metformin-cimetidine drug interaction trials, with a 60% increase in peak metformin plasma and whole blood concentrations and a 40% increase in plasma and whole blood metformin AUC. There was no change in elimination half-life in the single-dose trial. Metformin had no effect on cimetidine pharmacokinetics. [See Warnings and Precautions (5.1) and Drug Interactions (7.2).]
Furosemide: A single-dose, metformin-furosemide drug interaction trial in healthy subjects demonstrated that pharmacokinetic parameters of both compounds were affected by coadministration. Furosemide increased the metformin plasma and blood Cmax by 22% and blood AUC by 15%, without any significant change in metformin renal clearance. When administered with metformin, the Cmax and AUC of furosemide were 31% and 12% smaller, respectively, than when administered alone, and the terminal half-life was decreased by 32%, without any significant change in furosemide renal clearance. No information is available about the interaction of metformin and furosemide when coadministered chronically.
Nifedipine: A single-dose, metformin-nifedipine drug interaction trial in normal healthy volunteers demonstrated that coadministration of nifedipine increased plasma metformin Cmax and AUC by 20% and 9%, respectively, and increased the amount excreted in the urine. Tmax and half-life were unaffected. Nifedipine appears to enhance the absorption of metformin. Metformin had minimal effects on nifedipine.
Other: Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid.
In healthy volunteers, the pharmacokinetics of metformin and propranolol and metformin and ibuprofen were not affected when coadministered in single-dose interaction trials.
Metformin is negligibly bound to plasma proteins and is therefore, less likely to interact with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and probenecid.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted with AVANDAMET. The following data are based on findings in studies performed with rosiglitazone or metformin individually.
Rosiglitazone: A 2-year carcinogenicity study was conducted in Charles River CD-1 mice at doses of 0.4, 1.5, and 6 mg/kg/day in the diet (highest dose equivalent to approximately 12 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). Sprague-Dawley rats were dosed for 2 years by oral gavage at doses of 0.05, 0.3, and 2 mg/kg/day (highest dose equivalent to approximately 10 and 20 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET for male and female rats, respectively).
Rosiglitazone was not carcinogenic in the mouse. There was an increase in incidence of adipose hyperplasia in the mouse at doses ≥1.5 mg/kg/day (approximately 2 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). In rats, there was a significant increase in the incidence of benign adipose tissue tumors (lipomas) at doses ≥0.3 mg/kg/day (approximately 2 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). These proliferative changes in both species are considered due to the persistent pharmacological overstimulation of adipose tissue.
Rosiglitazone was not mutagenic or clastogenic in the in vitro bacterial assays for gene mutation, the in vitro chromosome aberration test in human lymphocytes, the in vivo mouse micronucleus test, and the in vivo/in vitro rat UDS assay. There was a small (about 2-fold) increase in mutation in the in vitro mouse lymphoma assay in the presence of metabolic activation.
Rosiglitazone had no effects on mating or fertility of male rats given up to 40 mg/kg/day (approximately 116 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). Rosiglitazone altered estrous cyclicity (2 mg/kg/day) and reduced fertility (40 mg/kg/day) of female rats in association with lower plasma levels of progesterone and estradiol (approximately 20 and 200 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively). No such effects were noted at 0.2 mg/kg/day (approximately 3 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET). In juvenile rats dosed from 27 days of age through to sexual maturity (at up to 40 mg/kg/day), there was no effect on male reproductive performance, or on estrous cyclicity, mating performance or pregnancy incidence in females (approximately 68 times human AUC at the maximum recommended daily dose of rosiglitazone). In monkeys, rosiglitazone (0.6 and 4.6 mg/kg/day; approximately 3 and 15 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively) diminished the follicular phase rise in serum estradiol with consequential reduction in the luteinizing hormone surge, lower luteal phase progesterone levels, and amenorrhea. The mechanism for these effects appears to be direct inhibition of ovarian steroidogenesis.
Metformin: Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1,500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommended human daily dose of 2,000 mg of the metformin component of AVANDAMET based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administrated at doses as high as 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose of the metformin component of AVANDAMET based on body surface area comparisons.
13.2 Animal Toxicology
Heart weights were increased in mice (3 mg/kg/day), rats (5 mg/kg/day), and dogs (2 mg/kg/day) with rosiglitazone treatments (approximately 5, 22, and 2 times human AUC at the maximum recommended human daily dose of the rosiglitazone component of AVANDAMET, respectively). Effects in juvenile rats were consistent with those seen in adults. Morphometric measurement indicated that there was hypertrophy in cardiac ventricular tissues, which may be due to increased heart work as a result of plasma volume expansion.
-
14 CLINICAL STUDIES
AVANDAMET was not studied in patients previously treated with metformin monotherapy; however, the combination of rosiglitazone and metformin was compared to rosiglitazone and metformin monotherapies in clinical trials. Bioequivalence between AVANDAMET and coadministered rosiglitazone tablets and metformin tablets has been demonstrated [see Clinical Pharmacology (12.3)].
A total of 670 patients with type 2 diabetes participated in two 26-week, randomized, double-blind, placebo/active-controlled trials designed to assess the efficacy of rosiglitazone in combination with metformin. Rosiglitazone, administered in either once-daily or twice-daily dosing regimens, was added to the therapy of patients who were inadequately controlled on 2.5 grams/day of metformin.
In one trial, patients inadequately controlled on 2.5 grams/day of metformin (mean baseline FPG 216 mg/dL and mean baseline HbA1c 8.8%) were randomized to receive rosiglitazone 4 mg once daily, rosiglitazone 8 mg once daily, or placebo in addition to metformin. A statistically significant improvement in FPG and HbA1c was observed in patients treated with the combinations of metformin and rosiglitazone 4 mg once daily and rosiglitazone 8 mg once daily, versus patients continued on metformin alone (see Table 11).
Table 11. Glycemic Parameters in a 26-Week Trial of Rosiglitazone Added to Metformin Therapy Metformin Rosiglitazone
4 mg once daily + metforminRosiglitazone
8 mg once daily + metforminN 113 116 110 FPG (mg/dL) Baseline (mean) 214 215 220 Change from baseline (mean) 6 -33 -48 Difference from metformin alone (adjusted mean) -40a -53a % of patients with ≥30 mg/dL decrease from baseline 20% 45% 61% HbA1c (%) Baseline (mean) 8.6 8.9 8.9 Change from baseline (mean) 0.5 -0.6 -0.8 Difference from metformin alone (adjusted mean) -1.0a -1.2a % of patients with HbA1c ≥0.7% decrease from baseline 11% 45% 52% aP <0.0001 compared to metformin.
In a second 26-week trial, patients with type 2 diabetes inadequately controlled on 2.5 grams/day of metformin who were randomized to receive the combination of rosiglitazone 4 mg twice daily and metformin (N = 105) showed a statistically significant improvement in glycemic control with a mean treatment effect for FPG of -56 mg/dL and a mean treatment effect for HbA1c of -0.8% over metformin alone. The combination of metformin and rosiglitazone resulted in lower levels of FPG and HbA1c than either agent alone.
-
15 REFERENCES
- Food and Drug Administration Briefing Document. Joint meeting of the Endocrinologic and Metabolic Drugs and Drug Safety and Risk Management Advisory Committees. July 13-14, 2010.
- Winkelmayer WC, et al. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch Intern Med 2008;168(21):2368-2375.
- Juurlink DN, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 2009; 339.
- Graham DJ, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. JAMA 2010;304:411-418.
- Wertz DA, et al. Risk of cardiovascular events and all-cause mortality in patients with Thiazolidinediones in a managed-care population. Circ Cardiovasc Qual Outcomes 2010;3: 538-545.
- DREAM Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096-1105.
- Kahn S, et al. Glycemic durability of rosiglitazone, metformin or glyburide monotherapy. New England Journal of Medicine 2006, 355:2427-2443.
- Home P, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicenter, randomized, open-label trial. Lancet 2009, 373:2125-35.
- Dormandy J et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (Prospective Pioglitazone Clinical Trial in Macrovascular Events): a randomized controlled trial. Lancet 2005, 366:1279-89.
- Bilik D, et al. Thiazolidinediones, cardiovascular disease and cardiovascular mortality: translating research into action for diabetes (TRIAD). Pharmocoepidemiol Drug Saf 2010; 19: 715–721.
- Park JY, Kim KA, Kang MH, et al. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther 2004;75:157-162.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each film-coated oval tablet contains rosiglitazone as the maleate and metformin hydrochloride as follows:
2 mg/500 mg – pale pink, tablet, debossed with gsk on one side and 2/500 on the other.
4 mg/500 mg – orange, tablet, debossed with gsk on one side and 4/500 on the other.
2 mg/1,000 mg – yellow, tablet, debossed with gsk on one side and 2/1000 on the other.
4 mg/1,000 mg – pink, tablet, debossed with gsk on one side and 4/1000 on the other.
2 mg/500 mg
Bottles of 20
NDC: 54868-4965-1
Bottles of 30
NDC: 54868-4965-2
Bottles of 60
NDC: 54868-4965-0
4 mg/500 mg
Bottles of 60
NDC: 54868-5157-0
2 mg/1,000 mg
Bottles of 60
NDC: 54868-5376-0
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). Dispense in a tight, light-resistant container. -
17 PATIENT COUNSELING INFORMATION
See Medication Guide.
17.1 Patient Advice
There are multiple medications available to treat type 2 diabetes. The benefits and risks of each available diabetes medication should be taken into account when choosing a particular diabetes medication for a given patient.
Patients should be informed of the risks and benefits of AVANDAMET. AVANDAMET should only be taken by adults with type 2 diabetes who are already taking rosiglitazone, or who are not already taking rosiglitazone and are unable to achieve adequate glycemic control on other diabetes medications, and, in consultation with their healthcare provider, have decided not to take pioglitazone (ACTOS) or pioglitazone-containing medications (ACTOPLUS MET, ACTOPLUS MET XR, DUETACT) for medical reasons.
Patients should be informed of the following:
- The risks of lactic acidosis, its symptoms, and conditions that predispose to its development, as noted in the WARNINGS and PRECAUTIONS sections, should be explained to patients. Patients should be advised to discontinue AVANDAMET immediately and to promptly notify their health practitioner if unexplained hyperventilation, myalgia, malaise, unusual somnolence, or other nonspecific symptoms occur. Once a patient is stabilized on any dose level of AVANDAMET, gastrointestinal symptoms, which are common during initiation of metformin therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
- Avoid excessive alcohol intake, either acute or chronic, while receiving AVANDAMET.
- AVANDAMET is not recommended for patients with symptomatic heart failure.
- Results of a set of clinical trials suggest that treatment with AVANDAMET is associated with an increased risk for myocardial infarction (heart attack), especially in patients taking insulin. Clinical trials have not shown any difference between rosiglitazone and comparator medications in overall mortality or CV-related mortality.
- AVANDAMET is not recommended for patients who are taking insulin.
- Management of type 2 diabetes should include diet control. Caloric restriction, weight loss, and exercise are essential for the proper treatment of the diabetic patient because they help improve insulin sensitivity. This is important not only in the primary treatment of type 2 diabetes but also in maintaining the efficacy of drug therapy.
- It is important to adhere to dietary instructions and to regularly have blood glucose, glycosylated hemoglobin (HbA1c), renal function, and hematologic parameters tested. It can take 2 weeks to see a reduction in blood glucose and 2 to 3 months to see the full effect of AVANDAMET.
- Blood will be drawn to check their liver function prior to the start of therapy and periodically thereafter per the clinical judgment of the healthcare professional. Patients with unexplained symptoms of nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine should immediately report these symptoms to their physician.
- Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on AVANDAMET should immediately report these symptoms to their physician.
- Therapy with AVANDAMET, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking AVANDAMET. Thus, adequate contraception in premenopausal women should be recommended. This possible effect has not been specifically investigated in clinical trials so the frequency of this occurrence is not known.
-
SPL UNCLASSIFIED SECTION
AVANDAMET is a registered trademark of GlaxoSmithKline.
GLUCOPHAGE is a registered trademark of Merck Santé S.A.S. (an associate of Merck KGaA of Darmstadt, Germany; licensed to Bristol-Myers Squibb Company). ACTOS, ACTOPLUS MET, ACTOPLUS MET XR, and DUETACT are registered trademarks of Takeda Pharmaceutical Company Limited.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2011, GlaxoSmithKline. All rights reserved.
February 2011
AVM:19PI
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
-
MEDICATION GUIDE
MEDICATION GUIDE
AVANDAMET® (ah-VAN-duh-met)
(rosiglitazone maleate and metformin hydrochloride) Tablets
Read this Medication Guide carefully before you start taking AVANDAMET and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about AVANDAMET, ask your doctor or pharmacist.
What is the most important information I should know about AVANDAMET?
AVANDAMET may cause serious side effects, including:
New or worse heart failure
- Rosiglitazone, one of the medicines in AVANDAMET, can cause your body to keep extra fluid (fluid retention), which leads to swelling (edema) and weight gain. Extra body fluid can make some heart problems worse or lead to heart failure. Heart failure means your heart does not pump blood well enough.
- If you have severe heart failure, you cannot start AVANDAMET.
- If you have heart failure with symptoms (such as shortness of breath or swelling), even if these symptoms are not severe, AVANDAMET may not be right for you.
Call your doctor right away if you have any of the following:
- swelling or fluid retention, especially in the ankles or legs
- shortness of breath or trouble breathing, especially when you lie down
- an unusually fast increase in weight
- unusual tiredness
Myocardial Infarction (“Heart Attack”)
Rosiglitazone, one of the medicines in AVANDAMET, may raise the risk of heart attack. The risk of having a heart attack may be higher in people who take AVANDAMET with insulin. Most people who take insulin should not also take AVANDAMET.
Symptoms of a heart attack can include the following:
- chest discomfort in the center of your chest that lasts for more than a few minutes, or that goes away or comes back
- chest discomfort that feels like uncomfortable pressure, squeezing, fullness or pain
- pain or discomfort in your arms, back, neck, jaw or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Call your doctor or go to the nearest hospital emergency room right away if you think you are having a heart attack.
People with diabetes have a greater risk for heart problems. It is important to work with your doctor to manage other conditions, such as high blood pressure or high cholesterol.
Lactic acidosis
Metformin, one of the medicines in AVANDAMET, can cause a rare but serious condition called lactic acidosis (a build-up of an acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.
Most people who have had lactic acidosis with metformin have other things that, combined with the metformin, led to the lactic acidosis. Tell your doctor if you have any of the following, because you have a higher chance for getting lactic acidosis with AVANDAMET if you:
- have kidney problems or your kidneys are affected by certain X-ray tests that use injectable dye. People with kidney problems should not take AVANDAMET.
- have liver problems
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have surgery
- have a heart attack, severe infection, or stroke
- are 80 years of age or older, and your kidneys are not working properly
The best way to keep from having a problem with lactic acidosis from metformin is to tell your doctor if you have any of the problems in the list above. Your doctor may decide to stop your AVANDAMET for a while if you have any of these things.
Lactic acidosis can be hard to diagnose early, because the early symptoms could seem like the symptoms of many other health problems besides lactic acidosis. You should call your doctor right away if you get the following symptoms, which could be signs of lactic acidosis:
- you feel very weak or tired
- you have unusual (not normal) muscle pain
- you have stomach pains
- you have trouble breathing
- you feel dizzy or lightheaded
- you have a slow or irregular heartbeat
AVANDAMET can have other serious side effects. Be sure to read the section below “What are possible side effects of AVANDAMET?”.
What is AVANDAMET?
AVANDAMET contains two prescription medicines for treating diabetes, rosiglitazone maleate (AVANDIA) and metformin hydrochloride. AVANDAMET is used, with diet and exercise, to treat certain adults with type 2 (“adult-onset” or “non-insulin dependent”) diabetes (“high blood sugar”) who are:
- already taking rosiglitazone or rosiglitazone-containing products
- unable to control their blood sugar on other diabetes medicines, and after talking with their doctor have decided not to take pioglitazone (ACTOS) or pioglitazone-containing products (ACTOPLUS MET, ACTOPLUS MET XR, DUETACT)
Metformin works mainly by decreasing the production of sugar by your liver. Rosiglitazone helps your body respond better to its natural insulin and does not cause your body to make more insulin. These medicines work together to help control your blood sugar. AVANDAMET may be used alone or with other diabetes medicines.
AVANDAMET is not for people with type 1 diabetes mellitus or to treat a condition called diabetic ketoacidosis.
It is not known if AVANDAMET is safe and effective in children under 18 years old.
Who should not take AVANDAMET?
Do not take AVANDAMET if you:
- have kidney problems. Before you take AVANDAMET and while you take it, your doctor should test your blood to check for signs of kidney problems.
- have a condition known as metabolic acidosis, including diabetic ketoacidosis.
- are going to have an x-ray procedure with an injection of dyes (contrast agents) in your vein with a needle. Talk to your doctor about when to stop AVANDAMET and when to start it again.
Many people with heart failure should not start taking AVANDAMET. See “What should I tell my doctor before taking AVANDAMET?”.
What should I tell my doctor before taking AVANDAMET?
Before starting AVANDAMET, ask your doctor about what the choices are for diabetes medicines, and what the expected benefits and possible risks are for you in particular.
Before taking AVANDAMET, tell your doctor about all your medical conditions, including if you:
- have heart problems or heart failure
- have kidney problems
- have type 1 (“juvenile”) diabetes or had diabetic ketoacidosis. These conditions should be treated with insulin.
- are going to have dye injected into a vein for an X-ray, CAT scan, heart study, or other type of scanning
- drink a lot of alcohol (all the time or short binge drinking).
- develop a serious condition such as a heart attack, severe infection, or a stroke.
- are 80 years old or older. People who are over 80 years old should not take AVANDAMET unless their kidney function is checked and it is normal.
- have a type of diabetic eye disease called macular edema (swelling of the back of the eye).
- have liver problems. Your doctor should do blood tests to check your liver before you start taking AVANDAMET and during treatment as needed.
- had liver problems while taking REZULIN® (troglitazone), another medicine for diabetes.
- are pregnant or plan to become pregnant. AVANDAMET should not be used during pregnancy. It is not known if AVANDAMET can harm your unborn baby. You and your doctor should talk about the best way to control your diabetes during pregnancy. If you are a premenopausal woman (before the “change of life”) who does not have regular monthly periods, AVANDAMET may increase your chances of becoming pregnant. Talk to your doctor about birth control choices while taking AVANDAMET. Tell your doctor right away if you become pregnant while taking AVANDAMET.
- are breast-feeding or planning to breast-feed. It is not known if AVANDAMET passes into breast milk. You should not use AVANDAMET while breast-feeding.
Tell your doctor about all the medicines you take including prescription and non-prescription medicines, vitamins or herbal supplements. AVANDAMET and certain other medicines can affect each other and may lead to serious side effects including high or low blood sugar, or heart problems. Your doctor may need to change your dose of AVANDAMET or your other medicines. Especially tell your doctor if you take:
- insulin.
- any medicines for high blood pressure, high cholesterol or heart failure, or for prevention of heart disease or stroke.
Know the medicines you take. Keep a list of all your medicines and show it to your doctor and pharmacist before you start a new medicine. They will tell you if it is alright to take AVANDAMET with other medicines.
How should I take AVANDAMET?
- Take AVANDAMET exactly as prescribed. Your doctor may need to change your dose until your blood sugar is better controlled.
- AVANDAMET should be taken by mouth and with meals.
- AVANDAMET may be prescribed alone or with other diabetes medicines. This will depend on how well your blood sugar is controlled.
- It can take 2 weeks for AVANDAMET to start lowering your blood sugar. It may take 2 to 3 months to see the full effect on your blood sugar level.
- If you miss a dose of AVANDAMET, take it as soon as you remember, unless it is time to take your next dose. Take your next dose at the usual time. Do not take double doses to make up for a missed dose.
- If you take too much AVANDAMET, call your doctor or poison control center right away.
- Test your blood sugar regularly as your doctor tells you.
- Diet and exercise can help your body use its blood sugar better. It is important to stay on your recommended diet, lose extra weight, and get regular exercise while taking AVANDAMET.
- Your doctor should do blood tests to check your liver and kidneys before you start AVANDAMET and during treatment as needed. Your doctor should also do regular blood sugar tests (for example, “A1C”) to monitor your response to AVANDAMET.
There may be times when you will need to stop taking AVANDAMET for a short time. Tell your doctor if you:
- are sick with severe vomiting, diarrhea or fever, or if you drink a much lower amount of liquid than normal.
- are going to have dye injected into a vein for an X-ray, CAT scan, heart study or other type of scanning.
- plan to have surgery.
What should I avoid while taking AVANDAMET?
Do not drink a lot of alcohol while taking AVANDAMET. This means you should not “binge drink”, and you should not drink a lot of alcohol on a regular basis. Drinking a lot of alcohol can increase the chance of getting lactic acidosis.
What are possible side effects of AVANDAMET?
AVANDAMET may cause serious side effects, including:
- New or worse heart failure. See “What is the most important information I should know about AVANDAMET?”.
- Heart attack. See “What is the most important information I should know about AVANDAMET?”.
- Swelling (edema). AVANDAMET can cause swelling due to fluid retention. See “What is the most important information I should know about AVANDAMET?”.
- Weight gain. Rosiglitazone, one of the medicines in AVANDAMET, can cause weight gain that may be due to fluid retention or extra body fat. Metformin, the other medicine in AVANDAMET, can cause weight loss. There is little change in weight with AVANDAMET. Weight gain can be a serious problem for people with certain conditions including heart problems. See “What is the most important information I should know about AVANDAMET?”
-
Liver problems. It is important for your liver to be working normally when you take AVANDAMET. Your doctor should do blood tests to check your liver before you start taking AVANDAMET and during treatment as needed. Call your doctor right away if you have unexplained symptoms such as:
- nausea or vomiting
- stomach pain
- unusual or unexplained tiredness
- loss of appetite
- dark urine
- yellowing of your skin or the whites of your eyes.
- Macular edema (a diabetic eye disease with swelling in the back of the eye). Tell your doctor right away if you have any changes in your vision. Your doctor should check your eyes regularly. Very rarely, some people have had vision changes due to swelling in the back of the eye while taking rosiglitazone, one of the medicines in AVANDAMET.
- Fractures (broken bones), usually in the hand, upper arm or foot. Talk to your doctor for advice on how to keep your bones healthy.
- Low red blood cell count (anemia).
- Low blood sugar(hypoglycemia). Lightheadedness, dizziness, shakiness or hunger may mean that your blood sugar is too low. This can happen if you skip meals, if you use another medicine that lowers blood sugar, or if you have certain medical problems. Call your doctor if low blood sugar levels are a problem for you.
- Ovulation (release of egg from an ovary in a woman) leading to pregnancy. Ovulation may happen in premenopausal women who do not have regular monthly periods. This can increase the chance of pregnancy. See “What should I tell my doctor before taking AVANDAMET?”.
Common side effects of AVANDAMET include:
- Diarrhea, nausea, and upset stomach. These side effects usually happen during the first few weeks of treatment. Taking AVANDAMET with food can help lessen these side effects. If you have unusual or unexpected stomach problems, talk with your doctor. Stomach problems that start up later during treatment with AVANDAMET may be a sign of something more serious and should be discussed with your doctor.
- Cold-like symptoms
- Headache
- Joint aches
- Dizziness
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store AVANDAMET?
- Store AVANDAMET at room temperature, 59° to 86°F (15° to 30°C).
- Keep AVANDAMET in the container it comes in. Keep the container closed tightly.
- Safely, throw away AVANDAMET that is out of date or no longer needed.
Keep AVANDAMET and all medicines out of the reach of children.
General information about AVANDAMET
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use AVANDAMET for a condition for which it was not prescribed. Do not give AVANDAMET to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes important information about AVANDAMET. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about AVANDAMET that is written for healthcare professionals. You can also find out more about AVANDAMET by calling 1-888-825-5249.
What are the ingredients in AVANDAMET?
Active Ingredients: Rosiglitazone maleate and metformin hydrochloride
Inactive Ingredients: Hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone 29-32, sodium starch glycolate, titanium dioxide, and 1 or more of the following: Red and yellow iron oxides.
Always check to make sure that the medicine you are taking is the correct one. AVANDAMET tablets are oval and look like this:
- 2 mg/500 mg – pale pink, with “gsk” on one side and “2/500” on the other.
- 4 mg/500 mg – orange, with “gsk” on one side and “4/500” on the other
- 2 mg/1,000 mg – yellow, with “gsk” on one side and “2/1000” on the other
- 4 mg/1,000 mg – pink, with “gsk” on one side and “4/1000” on the other
AVANDAMET is a registered trademark of GlaxoSmithKline.
The other brands listed are trademarks of their respective owners and are not trademarks of GlaxoSmithKline. The makers of these brands are not affiliated with and do not endorse GlaxoSmithKline or its products.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2011, GlaxoSmithKline. All rights reserved.
February 2011
AVM:3MG
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel

Avandamet®
Rosiglitazone maleate/metformin HCl
Tablets
2 mg/500 mg
Rx only
Federal Law requires dispensing of AVANDAMET® with the Medication Guide provided with this Bottle.
Store at 25oC (77oF); excursions permitted to 15oC-30oC (59oF-86oF).
Each tablet contains rosiglitazone maleate equivalent to 2 mg rosiglitazone and 500 mg metformin hydrochloride.
Dispense in a tight, light-resistant container.
Dosage: See accompanying prescribing information.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel

Avandamet®
Rosiglitazone maleate/metformin HCl
Tablets
2 mg/1000 mg
Rx only
Federal Law requires dispensing of AVANDAMET® with the Medication Guide provided with this Bottle.
Store at 25oC (77oF); excursions permitted to 15oC-30oC (59oF-86oF).
Each tablet contains rosiglitazone maleate equivalent to 2 mg rosiglitazone and 1000 mg metformin hydrochloride.
Dispense in a tight, light-resistant container.
Dosage: See accompanying prescribing information.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel

Avandamet®
Rosiglitazone maleate/metformin HCl
Tablets
4 mg/500 mg
60 Tablets
Rx only
Federal Law requires dispensing of AVANDAMET® with the Medication Guide provided with this Bottle.
Store at 25oC (77oF); excursions permitted to 15oC-30oC (59oF-86oF).
Each tablet contains rosiglitazone maleate equivalent to 4 mg rosiglitazone and 500 mg metformin hydrochloride.
Dispense in a tight, light-resistant container.
Dosage: See accompanying prescribing information.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel

Avandamet®
Rosiglitazone maleate/metformin HCl
Tablets
4 mg/1000 mg
60 Tablets
Rx only
Federal Law requires dispensing of AVANDAMET® with the Medication Guide provided with this Bottle.
Store at 25oC (77oF); excursions permitted to 15oC-30oC (59oF-86oF).
Each tablet contains rosiglitazone maleate equivalent to 4 mg rosiglitazone and 1000 mg metformin hydrochloride.
Dispense in a tight, light-resistant container.
Dosage: See accompanying prescribing information.
-
INGREDIENTS AND APPEARANCE
AVANDAMET
rosiglitazone maleate and metformin hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54868-4965(NDC: 0007-3167) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSIGLITAZONE MALEATE (UNII: KX2339DP44) (ROSIGLITAZONE - UNII:05V02F2KDG) ROSIGLITAZONE 2 mg METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K29/32 (UNII: 390RMW2PEQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK (pale pink) Score no score Shape OVAL Size 15mm Flavor Imprint Code gsk;2;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54868-4965-0 60 in 1 BOTTLE 2 NDC: 54868-4965-1 20 in 1 BOTTLE 3 NDC: 54868-4965-2 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021410 12/04/2003 AVANDAMET
rosiglitazone maleate and metformin hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54868-5157(NDC: 0007-3168) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSIGLITAZONE MALEATE (UNII: KX2339DP44) (ROSIGLITAZONE - UNII:05V02F2KDG) ROSIGLITAZONE 4 mg METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K29/32 (UNII: 390RMW2PEQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color ORANGE Score no score Shape OVAL Size 15mm Flavor Imprint Code gsk;4;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54868-5157-0 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021410 08/25/2006 AVANDAMET
rosiglitazone maleate and metformin hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54868-5376(NDC: 0007-3163) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSIGLITAZONE MALEATE (UNII: KX2339DP44) (ROSIGLITAZONE - UNII:05V02F2KDG) ROSIGLITAZONE 2 mg METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 1000 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K29/32 (UNII: 390RMW2PEQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape OVAL Size 19mm Flavor Imprint Code gsk;2;1000 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54868-5376-0 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021410 08/03/2005 AVANDAMET
rosiglitazone maleate and metformin hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54868-5262(NDC: 0007-3164) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSIGLITAZONE MALEATE (UNII: KX2339DP44) (ROSIGLITAZONE - UNII:05V02F2KDG) ROSIGLITAZONE 4 mg METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 1000 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K29/32 (UNII: 390RMW2PEQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape OVAL Size 19mm Flavor Imprint Code gsk;4;1000 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54868-5262-1 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021410 08/25/2005 06/30/2012 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel(54868-4965, 54868-5157, 54868-5376, 54868-5262) , repack(54868-4965, 54868-5157, 54868-5376, 54868-5262)
Trademark Results [AVANDAMET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AVANDAMET 78144579 2799327 Live/Registered |
SMITHKLINE BEECHAM (CORK) LIMITED 2002-07-17 |
 AVANDAMET 78063138 2807392 Live/Registered |
SMITHKLINE BEECHAM (CORK) LIMITED 2001-05-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.