DEHYDRATED ALCOHOL- alcohol injection, solution
DEHYDRATED ALCOHOL by
Drug Labeling and Warnings
DEHYDRATED ALCOHOL by is a Prescription medication manufactured, distributed, or labeled by Accord Healthcare, Inc., Maiva Pharma Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEHYDRATED ALCOHOL INJECTION safely and effectively. See full prescribing information for DEHYDRATED ALCOHOL INJECTION.
DEHYDRATED ALCOHOL injection, for cardiac septal branch intra-arterial use
Initial U.S. Approval: 1946INDICATIONS AND USAGE
Dehydrated alcohol injection is an ablative agent indicated to induce controlled cardiac septal infarction to improve exercise capacity in adults with symptomatic hypertrophic obstructive cardiomyopathy who are not candidates for surgical myectomy. (1) (1)
DOSAGE AND ADMINISTRATION
Inject small volumes over 1 to 2 minutes percutaneously into septal arterial branches, using the minimal dose necessary to achieve the desired reduction in peak left ventricular outflow tract pressure gradient. (2.1)
In most situations, a dose of 1 mL to 2 mL is sufficient. The maximum dose that should be used in a single procedure is 5 mL. (2.1) (2)DOSAGE FORMS AND STRENGTHS
Injection: 5 mL of ethyl alcohol ≥ 99% by volume as a clear, colorless liquid in a single-dose glass vial. (3) (3)
CONTRAINDICATIONS
None (4) (4)
WARNINGS AND PRECAUTIONS
Transient heart block: Transient heart block is common at the time of injection. A temporary pacing wire is routinely inserted to mitigate transient heart block. (5.1)
Persistent heart block: Approximately 10% of complete heart block events become permanent and require placement of a permanent pacemaker. (5.1)
Remove the temporary pacemaker lead if no episode of high-degree atrioventricular block occurs. (5.1)
Monitor the patient for heart failure, chest pain, and arrhythmias several days after the procedure. (5.1, 5.2, 5.3) (5)ADVERSE REACTIONS
Adverse reactions include arrhythmias, including ventricular tachycardia and/or ventricular fibrillation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Accord Healthcare Inc. at 1-866-941-7875 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Dehydrated alcohol injection is not recommended during pregnancy. Maternal use is not expected to result in fetal exposure to the drug. (8.1)
The rate of heart blocks and dysrhythmia increased with age. (8.5) (7)Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Administration
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Heart Block
5.2 Myocardial Infarction
5.3 Ventricular Arrhythmia
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
13.2 Animal Pharmacology & OR Toxicology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS & USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
Use the minimum dose necessary to achieve the desired reduction in peak left ventricular outflow tract pressure gradient. Inject small volumes over 1 to 2 minutes percutaneously into septal arterial branches, guided by assessment of the gradient. In most situations, a dose of 1 mL to 2 mL is sufficient. The maximum dose of dehydrated alcohol injection that should be used in a single procedure is 5 mL.
2.2 Administration
Dehydrated alcohol injection should only be administered under the supervision of a qualified interventional cardiologist experienced in the percutaneous transluminal septal myocardial ablation procedure.
Inspect visually for particulate matter and discoloration prior to administration. Dehydrated alcohol injection should appear as a clear, colorless solution. Discard unused portion.
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Heart Block
Transient Heart Block
Transient heart block is common at the time of dehydrated alcohol, such as dehydrated alcohol injection, injection into a septal artery. Prior to the injection, a temporary pacing wire is routinely inserted into the apex of the right ventricle, usually via the femoral vein, to treat transient heart block. The pacing lead can be removed if no episode of high-degree atrioventricular block occurs, usually after several hours of observation following percutaneous transluminal septal myocardial ablation.
Persistent Heart Block
Approximately 10% of complete heart block events become permanent and require placement of a permanent pacemaker following percutaneous transluminal septal myocardial ablation. Risk factors for permanent pacemaker dependency after septal ablation include a baseline PQ interval >160 ms, baseline minimum heart rate < 50 bpm, baseline left ventricular outflow gradient >70 mmHg, maximum QRS during the first 48 hours > 155 ms, 3rd degree atrio-ventricular block occurring during the procedure, and no clinical recovery between 12 to 48 hours after the procedure.5.2 Myocardial Infarction
Injection of dehydrated alcohol is intended to create a controlled myocardial infarction for therapeutic purposes. However, excessive myocardial necrosis and subsequent heart failure have been reported. Factors increasing the risk of excessive tissue necrosis include higher volume of alcohol used and a higher number of septal branches injected to reduce the left ventricular outflow tract gradient.
-
6 ADVERSE REACTIONS
Heart block [ see Warnings and precautions (5.1)]
The following other adverse reactions associated with percutaneous transluminal septal myocardial ablation with the use of dehydrated alcohol, such as dehydrated alcohol injection, were identified in the literature: Ventricular tachycardia and ventricular fibrillation. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The concentrations of alcohol in blood after PTSMA were not measured, but dehydrated alcohol injection is not expected to increase significantly the systemic concentrations of endogenous alcohol following administration into a septal artery during percutaneous transluminal septal myocardial ablation. Maternal use is not expected to result in fetal exposure to the drug.
Clinical Considerations
Dehydrated alcohol injection for percutaneous transluminal septal myocardial ablation has not been evaluated in pregnant women and is not recommended during pregnancy. When possible, the percutaneous transluminal septal myocardial ablation procedure should be postponed in women until the postpartum period.
Data
Animal reproduction studies have shown an adverse effect on the fetus and chronic fetal alcohol exposure is known to cause developmental defects in human. The developmental effects of acute ethanol exposure, such as from percutaneous transluminal septal myocardial ablation, have not been studied in pregnant or lactating women.8.2 Lactation
Dehydrated alcohol injection is not expected to increase significantly the systemic concentrations of endogenous alcohol following administration into a septal artery during percutaneous transluminal septal myocardial ablation and breastfeeding is not expected to result in exposure of the child to the drug.
8.5 Geriatric Use
A comparison of the outcomes in patients with hypertrophic obstructive cardiomyopathy in patients < 60 years old and in patients ≥ 60 years old showed similar improvement in exercise capacity after ablation. The rate of heart blocks and dysrhythmia increased with age. Permanent pacemaker dependency increased to 34% in patients > 60 years old.
- 10 OVERDOSAGE
-
11 DESCRIPTION
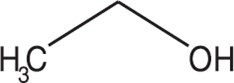
Dehydrated alcohol injection, USP is a sterile, preservative free solution of ≥ 99% by volume ethyl alcohol and no excipients. Dehydrated alcohol injection, USP is for cardiac septal branch intra-arterial use. It has a molecular formula of C 2H 6O and a molecular weight of 46.07.
Dehydrated alcohol injection, USP is a potent tissue toxin. Ethanol is a clear, colorless, volatile, and flammable liquid miscible with water. It has the following structural formula:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dehydrated alcohol is a tissue toxin that produces a myocardial infarction when injected through an intra-arterial catheter into a target septal vessel, which causes the hypertrophied septum to thin.
12.2 Pharmacodynamics
A dose independent, approximate 70% reduction of the peak pressure gradient across left ventricular outflow tract is observed after injection of alcohol volumes in the range of 1 to 4 mL. Remodeling contributes about 20% to the 70% total reduction in peak pressure gradient across the left ventricular outflow tract measured 12 months after septal ablation. Other markers, such as infarct size or peak concentration of creatine kinase-MB (CK-MB), in contrast to peak pressure gradient across the left ventricular outflow tract, vary in proportion to the injected alcohol volume in the 1 to 4 mL range.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Ethanol (of alcohol beverages) was added to Group 1 International Agency for Research on Cancer (IARC) Carcinogenicity Ratings (IARC monographs). Substances in this group are either carcinogenic to humans, or there is sufficient evidence of carcinogenicity in experimental animals and strong evidence in exposed humans that the substance acts through a relevant mechanism of carcinogenicity. Alcohol consumption has been associated with various cancers, including liver, esophageal, breast, prostate, and colorectal cancer. Since dehydrated alcohol injection is not expected to reach the systemic circulation following administration into a septal artery during percutaneous transluminal septal myocardial ablation, the recommended clinical use of the drug product is not expected to have carcinogenic risk in patients.
Literature reports suggest that ethanol is not mutagenic in the in vitro bacterial reverse mutation (Ames) assay or in vitro chromosomal aberration assays. Ethanol is metabolized to acetaldehyde, which is a known mutagen.
There are no data from either animal or human studies regarding potential for the impairment of fertility. - 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
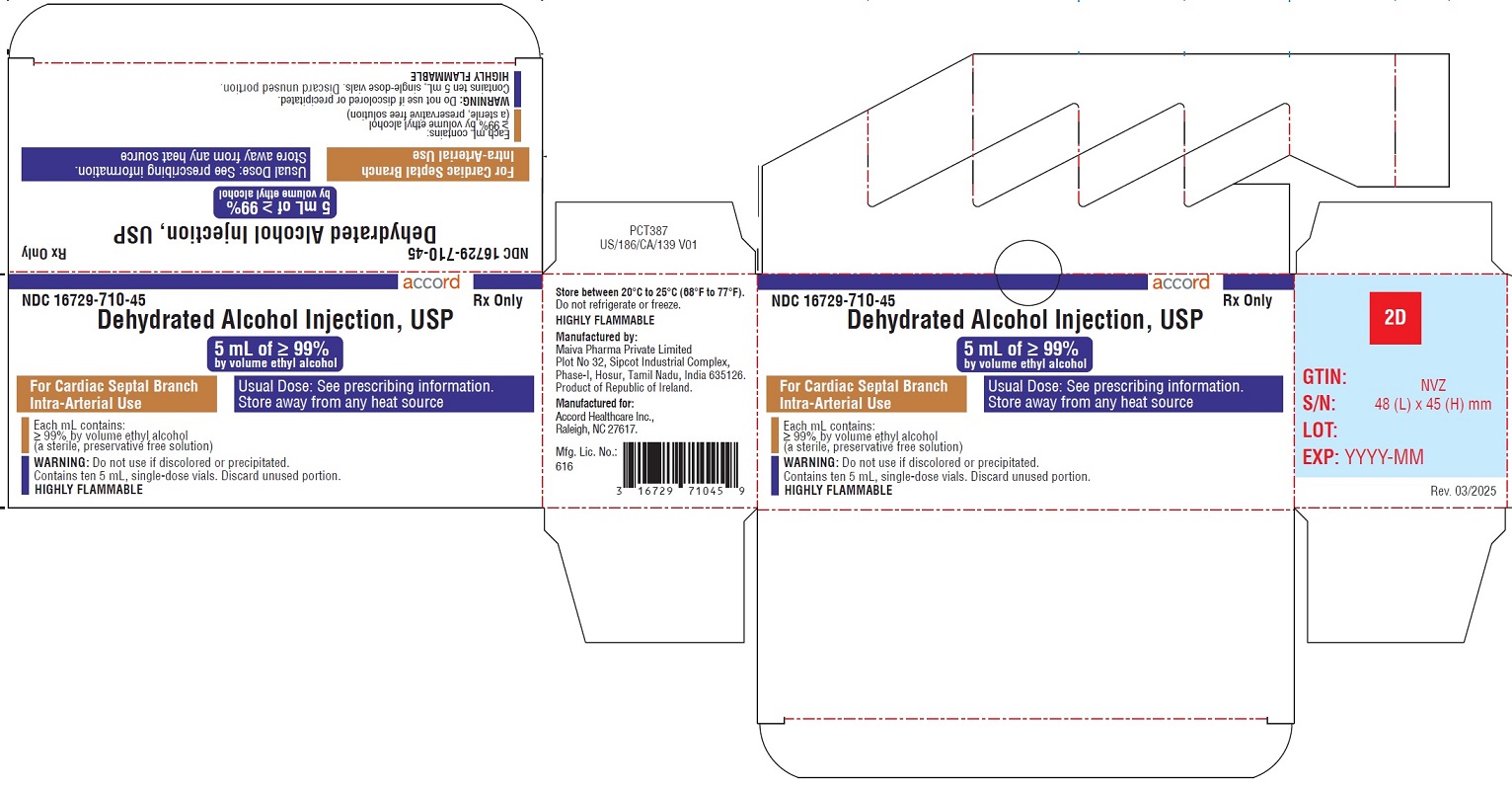
Dehydrated alcohol injection, USP is a clear, colorless liquid supplied in clear, glass, single-dose vials. Each mL contains ≥ 99% by volume ethyl alcohol.
NDC Number Package Size 16729-710-45 10 x 5 mL Single-Dose Vials Store at room temperature, between 20°C and 25°C (68°F and 77°F). Do not refrigerate or freeze. Highly flammable, store away from any heat source.
Manufactured for:
Accord Healthcare, Inc.,
8041 Arco Corporate Drive,
Suite 200, Raleigh,
NC 27617, USA.Manufactured By:
Maiva Pharma Private Limited
Plot No 32, Sipcot Industrial Complex,
Phase-I, Hosur, Tamil Nadu, India 635126.Product of Republic of Ireland.
Issued March 2025
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEHYDRATED ALCOHOL
alcohol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16729-710 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16729-710-45 10 in 1 CARTON 06/25/2025 1 NDC: 16729-710-31 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217845 06/25/2025 Labeler - Accord Healthcare, Inc. (604222237) Establishment Name Address ID/FEI Business Operations Maiva Pharma Private Limited 725656438 analysis(16729-710) , label(16729-710) , manufacture(16729-710) , pack(16729-710)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.