Nasal Decolonization Swab by I2 Strategic Solutions / I2 Strategic Solutions LLC
Nasal Decolonization Swab by
Drug Labeling and Warnings
Nasal Decolonization Swab by is a Otc medication manufactured, distributed, or labeled by I2 Strategic Solutions, I2 Strategic Solutions LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
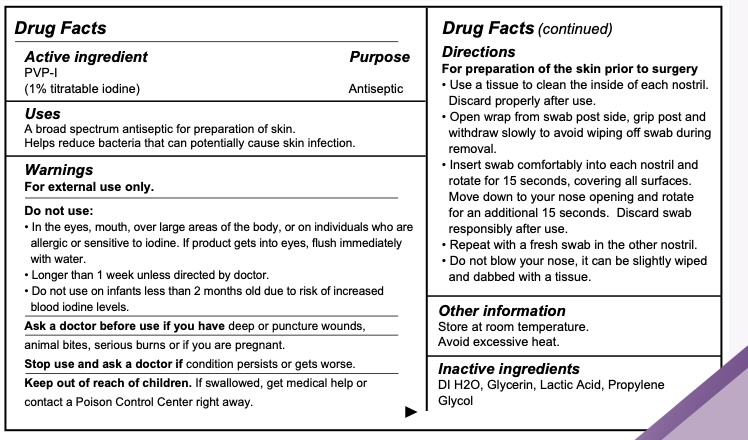
NASAL DECOLONIZATION SWAB- pvp-i solution liquid
I2 Strategic Solutions
----------
| NASAL DECOLONIZATION SWAB
pvp-i solution liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - I2 Strategic Solutions (118985323) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| I2 Strategic Solutions LLC | 118985323 | manufacture(83465-101) | |
Revised: 11/2024
Document Id: 2759946e-9831-9372-e063-6294a90aea47
Set id: 0827d95f-d136-2d96-e063-6394a90ae347
Version: 2
Effective Time: 20241120
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.