ORENCIA- abatacept injection, powder, lyophilized, for solution ORENCIA- abatacept injection, solution
ORENCIA by
Drug Labeling and Warnings
ORENCIA by is a Prescription medication manufactured, distributed, or labeled by E.R. Squibb & Sons, L.L.C.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ORENCIA safely and effectively. See full prescribing information for ORENCIA.
ORENCIA (abatacept) for injection, for intravenous use
ORENCIA (abatacept) injection, for subcutaneous use
Initial U.S. Approval: 2005INDICATIONS AND USAGE
ORENCIA is a selective T cell costimulation modulator indicated for:
Adult Rheumatoid Arthritis (RA) (1.1)- moderately to severely active RA in adults. ORENCIA may be used as monotherapy or concomitantly with DMARDs other than TNF antagonists (1.1).
Juvenile Idiopathic Arthritis (1.2)
- moderately to severely active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older. ORENCIA may be used as monotherapy or concomitantly with methotrexate (1.2).
Adult Psoriatic Arthritis (PsA) (1.3)
- active PsA in adults. (1.3)
Important Limitations of Use (1.4)
DOSAGE AND ADMINISTRATION
Intravenous Administration for Adult RA (2.1) and Adult PsA (2.3)
Body Weight of Patient Dose Number of Vials Less than 60 kg
500 mg
2
60 to 100 kg
750 mg
3
More than 100 kg
1000 mg
4
Subcutaneous Administration for Adult RA (2.1)
- Administer by subcutaneous injection once weekly with or without an intravenous loading dose. For patients initiating therapy with an intravenous loading dose, administer a single intravenous infusion (as per body weight categories above), followed by the first 125 mg subcutaneous injection given within a day of the intravenous infusion.
- Patients transitioning from ORENCIA intravenous therapy to subcutaneous administration should administer the first subcutaneous dose instead of the next scheduled intravenous dose.
Intravenous Administration for Juvenile Idiopathic Arthritis (2.2)
- Pediatric patients weighing less than 75 kg receive 10 mg/kg intravenously based on the patient's body weight. Pediatric patients weighing 75 kg or more should be administered ORENCIA following the adult intravenous dosing regimen, not to exceed a maximum dose of 1000 mg. Intravenous dosing has not been studied in patients younger than 6 years of age. (2.2)
Subcutaneous Administration for Juvenile Idiopathic Arthritis (2.2)
Body Weight of Patient Dose (once weekly) 10 to less than 25 kg
50 mg
25 to less than 50 kg
87.5 mg
50 kg or more
125 mg
Subcutaneous Administration for Adult PsA (2.3)
- Administer by subcutaneous injection once weekly without the need of an intravenous loading dose.
- Patients transitioning from ORENCIA intravenous therapy to subcutaneous administration should administer the first subcutaneous dose instead of the next scheduled intravenous dose.
General Dosing Information for Intravenous Administration (2.1 and 2.3)
- Administer as a 30-minute intravenous infusion (2.1, 2.2)
- Following initial dose, give at 2 and 4 weeks, then every 4 weeks (2.1)
- Prepare ORENCIA using only the silicone-free disposable syringe (2.3)
- Use only Sterile Water for Injection, USP to reconstitute the powder (2.3)
- The reconstituted and diluted product must be administered using a filter (2.3)
DOSAGE FORMS AND STRENGTHS
Intravenous Infusion
- For Injection: 250 mg lyophilized powder in a single-use vial for reconstitution and dilution prior to intravenous infusion (3)
Subcutaneous Injection
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
- Concomitant use with a TNF antagonist can increase the risk of infections and serious infections (5.1)
- Hypersensitivity, anaphylaxis, and anaphylactoid reactions (5.2)
- Patients with a history of recurrent infections or underlying conditions predisposing to infections may experience more infections (5.3, 8.5)
- Discontinue if a serious infection develops (5.3)
- Screen for latent TB infection prior to initiating therapy. Patients testing positive should be treated prior to initiating ORENCIA (5.3)
- Live vaccines should not be given concurrently or within 3 months of discontinuation (5.4)
- Patients with juvenile idiopathic arthritis should be brought up to date with all immunizations prior to ORENCIA therapy (5.4)
- Based on its mechanism of action, ORENCIA may blunt the effectiveness of some immunizations (5.4)
- COPD patients may develop more frequent respiratory adverse events (5.5)
ADVERSE REACTIONS
Most common adverse events (≥10%) are headache, upper respiratory tract infection, nasopharyngitis, and nausea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
- Pregnancy: Registry available. The data with ORENCIA use in pregnant women are insufficient to inform on drug-associated risk (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adult Rheumatoid Arthritis (RA)
1.2 Juvenile Idiopathic Arthritis
1.3 Adult Psoriatic Arthritis (PsA)
1.4 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Adult Rheumatoid Arthritis
2.2 Juvenile Idiopathic Arthritis
2.3 Adult Psoriatic Arthritis
2.4 Preparation and Administration Instructions for Intravenous Infusion
2.5 General Considerations for Subcutaneous Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Concomitant Use with TNF Antagonists
5.2 Hypersensitivity
5.3 Infections
5.4 Immunizations
5.5 Use in Patients with Chronic Obstructive Pulmonary Disease (COPD)
5.6 Immunosuppression
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience in Adult RA Patients Treated with Intravenous ORENCIA
6.2 Clinical Studies Experience in Adult RA Patients Treated with Subcutaneous ORENCIA
6.3 Clinical Studies Experience in Juvenile Idiopathic Arthritis Patients Treated with Intravenous ORENCIA
6.4 Clinical Studies Experience in Juvenile Idiopathic Arthritis Patients Treated with Subcutaneous ORENCIA
6.5 Clinical Studies Experience in Adult PsA Patients
6.6 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 TNF Antagonists
7.2 Other Biologic RA Therapy
7.3 Blood Glucose Testing
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adult Rheumatoid Arthritis
14.2 Juvenile Idiopathic Arthritis
14.3 Adult Psoriatic Arthritis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Adult Rheumatoid Arthritis (RA)
ORENCIA® is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active rheumatoid arthritis. ORENCIA may be used as monotherapy or concomitantly with disease-modifying antirheumatic drugs (DMARDs) other than tumor necrosis factor (TNF) antagonists.
1.2 Juvenile Idiopathic Arthritis
ORENCIA is indicated for reducing signs and symptoms in patients 2 years of age and older with moderately to severely active polyarticular juvenile idiopathic arthritis. ORENCIA may be used as monotherapy or concomitantly with methotrexate (MTX).
-
2 DOSAGE AND ADMINISTRATION
2.1 Adult Rheumatoid Arthritis
For adult patients with RA, ORENCIA may be administered as an intravenous infusion or as a subcutaneous injection.
ORENCIA may be used as monotherapy or concomitantly with DMARDs other than TNF antagonists.
Intravenous Dosing Regimen
ORENCIA lyophilized powder should be reconstituted and administered after dilution [see Dosage and Administration (2.3)] as a 30-minute intravenous infusion utilizing the weight range-based dosing specified in Table 1. Following the initial intravenous administration, an intravenous infusion should be given at 2 and 4 weeks after the first infusion and every 4 weeks thereafter.
Table 1: Dose of ORENCIA for Intravenous Infusion in Adult RA Patients Body Weight of Patient Dose Number of Vialsa a Each vial provides 250 mg of abatacept for administration. Less than 60 kg
500 mg
2
60 to 100 kg
750 mg
3
More than 100 kg
1000 mg
4
Subcutaneous Dosing Regimen
ORENCIA 125 mg in prefilled syringes or in ORENCIA ClickJect™ autoinjector should be administered by subcutaneous injection once weekly [see Dosage and Administration (2.4)] and may be initiated with or without an intravenous loading dose. For patients initiating therapy with an intravenous loading dose, ORENCIA should be initiated with a single intravenous infusion (as per body weight categories listed in Table 1), followed by the first 125 mg subcutaneous injection administered within a day of the intravenous infusion.
Patients transitioning from ORENCIA intravenous therapy to subcutaneous administration should administer the first subcutaneous dose instead of the next scheduled intravenous dose.
2.2 Juvenile Idiopathic Arthritis
For patients with juvenile idiopathic arthritis (JIA), ORENCIA may be administered as an intravenous infusion (6 years of age and older) or a subcutaneous injection (2 years of age and older). Intravenous dosing has not been studied in patients younger than 6 years of age.
ORENCIA may be used as monotherapy or concomitantly with methotrexate.
Intravenous Dosing Regimen
ORENCIA should be administered as a 30-minute intravenous infusion based on body weight. Pediatric patients with:
- body weight less than 75 kg should be administered ORENCIA at a dose of 10 mg/kg [see Dosage and Administration (2.3)].
- body weight of 75 kg or more should be administered ORENCIA following the adult intravenous dosing regimen (see Table 1), not to exceed a maximum dose of 1000 mg.
Following the initial administration, ORENCIA should be given at 2 and 4 weeks after the first infusion and every 4 weeks thereafter. Any unused portions in the vials must be immediately discarded.
Subcutaneous Dosing Regimen
ORENCIA for subcutaneous injection should be initiated without an intravenous loading dose and be administered utilizing the weight range-based dosing as specified in Table 2.
Table 2: Dose of ORENCIA for Subcutaneous Administration in Patients 2 Years of Age or Older with JIA Body Weight of Patient Dose (once weekly) 10 to less than 25 kg
50 mg
25 to less than 50 kg
87.5 mg
50 kg or more
125 mg
The safety and efficacy of ORENCIA ClickJect autoinjector for subcutaneous injection has not been studied in patients under 18 years of age.
2.3 Adult Psoriatic Arthritis
For adult patients with psoriatic arthritis, ORENCIA may be administered as an intravenous infusion (IV) or a subcutaneous (SC) injection.
ORENCIA can be used with or without non-biologic DMARDs.
Intravenous Dosing Regimen
ORENCIA IV should be administered as a 30-minute intravenous infusion utilizing the weight range-based dosing specified in Table 1. Following the initial intravenous administration, an intravenous infusion should be given at 2 and 4 weeks after the first infusion and every 4 weeks thereafter.
Subcutaneous Dosing Regimen
ORENCIA SC 125 mg should be administered by subcutaneous injection once weekly without the need for an intravenous loading dose.
Patients switching from ORENCIA intravenous therapy to subcutaneous administration should administer the first subcutaneous dose instead of the next scheduled intravenous dose.
2.4 Preparation and Administration Instructions for Intravenous Infusion
Use aseptic technique.
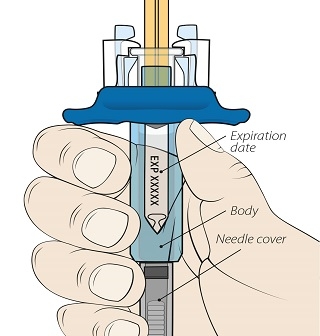
ORENCIA for Injection is provided as a lyophilized powder in preservative-free, single-use vials. Each ORENCIA vial provides 250 mg of abatacept for administration. The ORENCIA powder in each vial must be reconstituted with 10 mL of Sterile Water for Injection, USP, using only the silicone-free disposable syringe provided with each vial and an 18- to 21-gauge needle. After reconstitution, the concentration of abatacept in the vial will be 25 mg/mL. If the ORENCIA powder is accidentally reconstituted using a siliconized syringe, the solution may develop a few translucent particles. Discard any solutions prepared using siliconized syringes.
If the silicone-free disposable syringe is dropped or becomes contaminated, use a new silicone-free disposable syringe from inventory. For information on obtaining additional silicone-free disposable syringes, contact Bristol-Myers Squibb 1-800-ORENCIA.
- 1)
Use 10 mL of Sterile Water for Injection, USP to reconstitute the ORENCIA powder. To reconstitute the ORENCIA powder, remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of Sterile Water for Injection, USP, to the glass wall of the vial. Do not use the vial if the vacuum is not present. Rotate the vial with gentle swirling to minimize foam formation, until the contents are completely dissolved. Do not shake. Avoid prolonged or vigorous agitation.
- 2)
Upon complete dissolution of the lyophilized powder, the vial should be vented with a needle to dissipate any foam that may be present. After reconstitution, each milliliter will contain 25 mg (250 mg/10 mL). The solution should be clear and colorless to pale yellow. Do not use if opaque particles, discoloration, or other foreign particles are present.
- 3)
The reconstituted ORENCIA solution must be further diluted to 100 mL as follows. From a 100 mL infusion bag or bottle, withdraw a volume of 0.9% Sodium Chloride Injection, USP, equal to the volume of the reconstituted ORENCIA solution required for the patient’s dose. Slowly add the reconstituted ORENCIA solution into the infusion bag or bottle using the same silicone-free disposable syringe provided with each vial. Gently mix. Do not shake the bag or bottle. The final concentration of abatacept in the bag or bottle will depend upon the amount of drug added, but will be no more than 10 mg/mL. Any unused portions in the ORENCIA vial must be immediately discarded.
- 4)
Prior to administration, the ORENCIA solution should be inspected visually for particulate matter and discoloration. Discard the solution if any particulate matter or discoloration is observed.
- 5)
The entire, fully diluted ORENCIA solution should be administered over a period of 30 minutes and must be administered with an infusion set and a sterile, non-pyrogenic, low-protein-binding filter (pore size of 0.2 μm to 1.2 μm).
- 6)
The infusion of the fully diluted ORENCIA solution must be completed within 24 hours of reconstitution of the ORENCIA vials. The fully diluted ORENCIA solution may be stored at room temperature or refrigerated at 2°C to 8°C (36°F to 46°F) before use. Discard the fully diluted solution if not administered within 24 hours.
- 7)
ORENCIA should not be infused concomitantly in the same intravenous line with other agents. No physical or biochemical compatibility studies have been conducted to evaluate the coadministration of ORENCIA with other agents.
2.5 General Considerations for Subcutaneous Administration
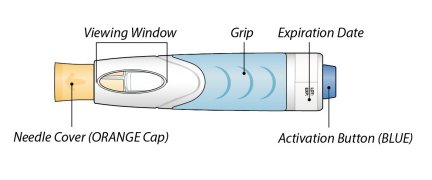
ORENCIA prefilled syringes and ORENCIA ClickJect autoinjectors are intended for subcutaneous use only and are not intended for intravenous infusion.
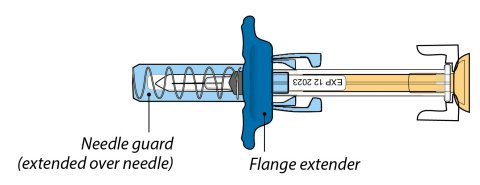
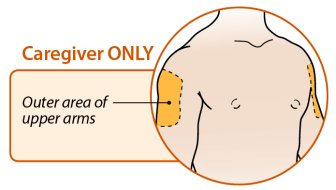
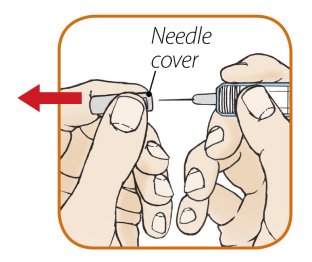
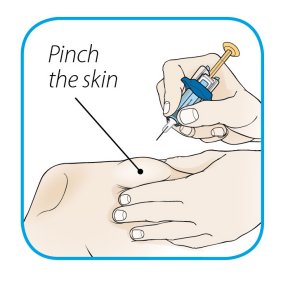
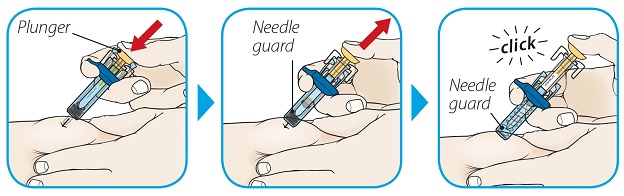
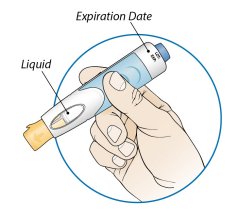
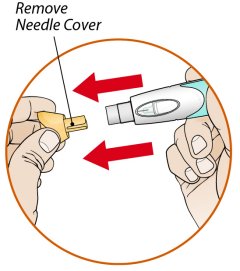
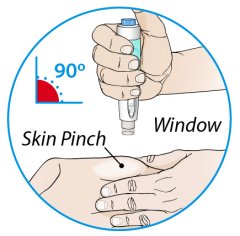
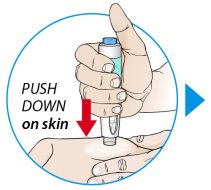
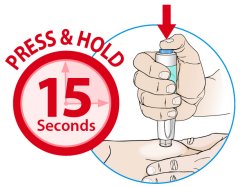
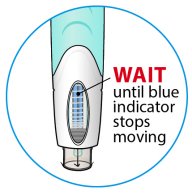
ORENCIA prefilled syringes and ORENCIA ClickJect autoinjectors are intended for use under the guidance of a physician or healthcare practitioner. After proper training in subcutaneous injection technique, a patient or caregiver may inject with ORENCIA if a physician/healthcare practitioner determines that it is appropriate. Patients and caregivers should be instructed to follow the directions provided in the Instructions for Use for additional details on medication administration.
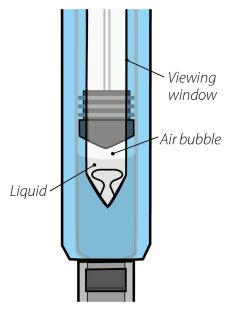
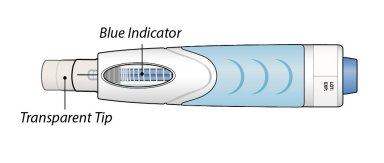
Inspect visually for particulate matter and discoloration prior to administration. Do not use ORENCIA prefilled syringes or ORENCIA ClickJect autoinjectors exhibiting particulate matter or discoloration. ORENCIA should be clear and colorless to pale yellow.
Patients using ORENCIA prefilled syringes and ORENCIA ClickJect autoinjectors for subcutaneous administration should be instructed to inject the full amount, which provides the proper dose of ORENCIA, according to the directions provided in the Instructions for Use.
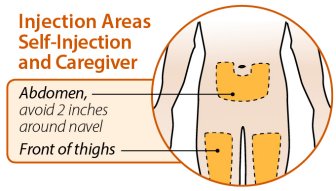
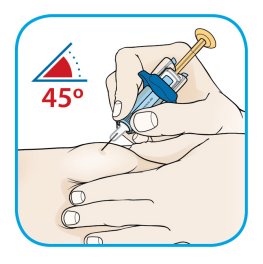
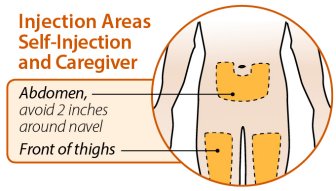
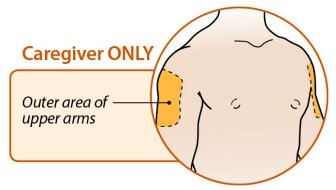
Injection sites should be rotated and injections should never be given into areas where the skin is tender, bruised, red, or hard.
-
3 DOSAGE FORMS AND STRENGTHS
- Intravenous Infusion
- For Injection: 250 mg lyophilized powder in a single-use vial
- Subcutaneous Injection
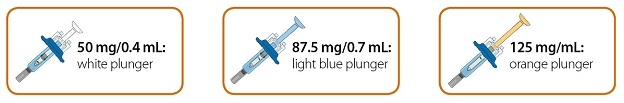
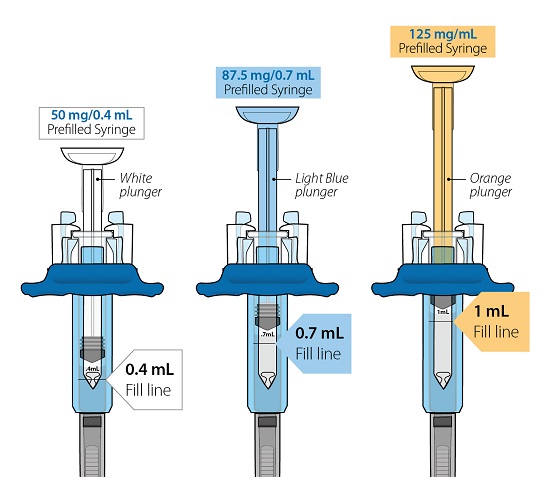
- Injection: 50 mg/0.4 mL, 87.5 mg/0.7 mL, and 125 mg/mL of a clear to slightly opalescent, colorless to pale-yellow solution in a single-dose prefilled glass syringe.
- Injection: 125 mg/mL of a clear to slightly opalescent, colorless to pale-yellow solution in a single-dose prefilled ClickJect autoinjector.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Concomitant Use with TNF Antagonists
In controlled clinical trials in patients with adult RA, patients receiving concomitant intravenous ORENCIA and TNF antagonist therapy experienced more infections (63%) and serious infections (4.4%) compared to patients treated with only TNF antagonists (43% and 0.8%, respectively) [see Adverse Reactions (6.1)]. These trials failed to demonstrate an important enhancement of efficacy with concomitant administration of ORENCIA with TNF antagonist; therefore, concurrent therapy with ORENCIA and a TNF antagonist is not recommended. While transitioning from TNF antagonist therapy to ORENCIA therapy, patients should be monitored for signs of infection.
5.2 Hypersensitivity
In clinical trials of 2688 adult RA patients treated with intravenous ORENCIA, there were two cases (<0.1%) of anaphylaxis or anaphylactoid reactions. Other reactions potentially associated with drug hypersensitivity, such as hypotension, urticaria, and dyspnea, each occurred in less than 0.9% of ORENCIA-treated patients. Of the 190 patients with juvenile idiopathic arthritis treated with ORENCIA in clinical trials, there was one case of a hypersensitivity reaction (0.5%). Appropriate medical support measures for the treatment of hypersensitivity reactions should be available for immediate use in the event of a reaction [see Adverse Reactions (6.1, 6.3)]. Anaphylaxis or anaphylactoid reactions can occur after the first infusion and can be life threatening. In postmarketing experience, a case of fatal anaphylaxis following the first infusion of ORENCIA has been reported. If an anaphylactic or other serious allergic reaction occurs, administration of ORENCIA should be stopped immediately with appropriate therapy instituted, and the use of ORENCIA should be permanently discontinued.
5.3 Infections
Serious infections, including sepsis and pneumonia, have been reported in patients receiving ORENCIA. Some of these infections have been fatal. Many of the serious infections have occurred in patients on concomitant immunosuppressive therapy which in addition to their underlying disease, could further predispose them to infection. Physicians should exercise caution when considering the use of ORENCIA in patients with a history of recurrent infections, underlying conditions which may predispose them to infections, or chronic, latent, or localized infections. Patients who develop a new infection while undergoing treatment with ORENCIA should be monitored closely. Administration of ORENCIA should be discontinued if a patient develops a serious infection [see Adverse Reactions (6.1)]. A higher rate of serious infections has been observed in adult RA patients treated with concurrent TNF antagonists and ORENCIA [see Warnings and Precautions (5.1)].
Prior to initiating immunomodulatory therapies, including ORENCIA, patients should be screened for latent tuberculosis infection with a tuberculin skin test. ORENCIA has not been studied in patients with a positive tuberculosis screen, and the safety of ORENCIA in individuals with latent tuberculosis infection is unknown. Patients testing positive in tuberculosis screening should be treated by standard medical practice prior to therapy with ORENCIA.
Antirheumatic therapies have been associated with hepatitis B reactivation. Therefore, screening for viral hepatitis should be performed in accordance with published guidelines before starting therapy with ORENCIA. In clinical studies with ORENCIA, patients who screened positive for hepatitis were excluded from study.
5.4 Immunizations
Live vaccines should not be given concurrently with ORENCIA or within 3 months of its discontinuation. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving ORENCIA. The efficacy of vaccination in patients receiving ORENCIA is not known. Based on its mechanism of action, ORENCIA may blunt the effectiveness of some immunizations.
It is recommended that patients with juvenile idiopathic arthritis be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating ORENCIA therapy.
5.5 Use in Patients with Chronic Obstructive Pulmonary Disease (COPD)
Adult COPD patients treated with ORENCIA developed adverse events more frequently than those treated with placebo, including COPD exacerbations, cough, rhonchi, and dyspnea. Use of ORENCIA in patients with RA and COPD should be undertaken with caution and such patients should be monitored for worsening of their respiratory status [see Adverse Reactions (6.1)].
5.6 Immunosuppression
The possibility exists for drugs inhibiting T cell activation, including ORENCIA, to affect host defenses against infections and malignancies since T cells mediate cellular immune responses. The impact of treatment with ORENCIA on the development and course of malignancies is not fully understood [see Adverse Reactions (6.1)]. In clinical trials in patients with adult RA, a higher rate of infections was seen in ORENCIA-treated patients compared to placebo [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not predict the rates observed in a broader patient population in clinical practice.
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to abatacept in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
6.1 Clinical Studies Experience in Adult RA Patients Treated with Intravenous ORENCIA
The data described herein reflect exposure to ORENCIA administered intravenously in patients with active RA in placebo-controlled studies (1955 patients with ORENCIA, 989 with placebo). The studies had either a double-blind, placebo-controlled period of 6 months (258 patients with ORENCIA, 133 with placebo) or 1 year (1697 patients with ORENCIA, 856 with placebo). A subset of these patients received concomitant biologic DMARD therapy, such as a TNF blocking agent (204 patients with ORENCIA, 134 with placebo).
The majority of patients in RA clinical studies received one or more of the following concomitant medications with ORENCIA: methotrexate, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, TNF blocking agents, azathioprine, chloroquine, gold, hydroxychloroquine, leflunomide, sulfasalazine, and anakinra.
The most serious adverse reactions were serious infections and malignancies.
The most commonly reported adverse events (occurring in ≥10% of patients treated with ORENCIA) were headache, upper respiratory tract infection, nasopharyngitis, and nausea.
The adverse events most frequently resulting in clinical intervention (interruption or discontinuation of ORENCIA) were due to infection. The most frequently reported infections resulting in dose interruption were upper respiratory tract infection (1.0%), bronchitis (0.7%), and herpes zoster (0.7%). The most frequent infections resulting in discontinuation were pneumonia (0.2%), localized infection (0.2%), and bronchitis (0.1%).
Infections
In the placebo-controlled trials, infections were reported in 54% of ORENCIA-treated patients and 48% of placebo-treated patients. The most commonly reported infections (reported in 5%-13% of patients) were upper respiratory tract infection, nasopharyngitis, sinusitis, urinary tract infection, influenza, and bronchitis. Other infections reported in fewer than 5% of patients at a higher frequency (>0.5%) with ORENCIA compared to placebo, were rhinitis, herpes simplex, and pneumonia [see Warnings and Precautions (5.3)].
Serious infections were reported in 3.0% of patients treated with ORENCIA and 1.9% of patients treated with placebo. The most common (0.2%-0.5%) serious infections reported with ORENCIA were pneumonia, cellulitis, urinary tract infection, bronchitis, diverticulitis, and acute pyelonephritis [see Warnings and Precautions (5.3)].
Malignancies
In the placebo-controlled portions of the clinical trials (1955 patients treated with ORENCIA for a median of 12 months), the overall frequencies of malignancies were similar in the ORENCIA- and placebo-treated patients (1.3% and 1.1%, respectively). However, more cases of lung cancer were observed in ORENCIA-treated patients (4, 0.2%) than placebo-treated patients (0). In the cumulative ORENCIA clinical trials (placebo-controlled and uncontrolled, open-label) a total of 8 cases of lung cancer (0.21 cases per 100 patient-years) and 4 lymphomas (0.10 cases per 100 patient-years) were observed in 2688 patients (3827 patient-years). The rate observed for lymphoma is approximately 3.5-fold higher than expected in an age- and gender-matched general population based on the National Cancer Institute's Surveillance, Epidemiology, and End Results Database. Patients with RA, particularly those with highly active disease, are at a higher risk for the development of lymphoma. Other malignancies included skin, breast, bile duct, bladder, cervical, endometrial, lymphoma, melanoma, myelodysplastic syndrome, ovarian, prostate, renal, thyroid, and uterine cancers [see Warnings and Precautions (5.6)]. The potential role of ORENCIA in the development of malignancies in humans is unknown.
Infusion-Related Reactions and Hypersensitivity Reactions
Acute infusion-related events (adverse reactions occurring within 1 hour of the start of the infusion) in Studies III, IV, and V [see Clinical Studies (14.1)] were more common in the ORENCIA-treated patients than the placebo patients (9% for ORENCIA, 6% for placebo). The most frequently reported events (1%-2%) were dizziness, headache, and hypertension.
Acute infusion-related events that were reported in >0.1% and ≤1% of patients treated with ORENCIA included cardiopulmonary symptoms, such as hypotension, increased blood pressure, and dyspnea; other symptoms included nausea, flushing, urticaria, cough, hypersensitivity, pruritus, rash, and wheezing. Most of these reactions were mild (68%) to moderate (28%). Fewer than 1% of ORENCIA-treated patients discontinued due to an acute infusion-related event. In controlled trials, 6 ORENCIA-treated patients compared to 2 placebo-treated patients discontinued study treatment due to acute infusion-related events.
In clinical trials of 2688 adult RA patients treated with intravenous ORENCIA, there were two cases (<0.1%) of anaphylaxis or anaphylactoid reactions. Other reactions potentially associated with drug hypersensitivity, such as hypotension, urticaria, and dyspnea, each occurred in less than 0.9% of ORENCIA-treated patients and generally occurred within 24 hours of ORENCIA infusion. Appropriate medical support measures for the treatment of hypersensitivity reactions should be available for immediate use in the event of a reaction [see Warnings and Precautions (5.2)].
Adverse Reactions in Patients with COPD
In Study V [see Clinical Studies (14.1)], there were 37 patients with chronic obstructive pulmonary disease (COPD) who were treated with ORENCIA and 17 COPD patients who were treated with placebo. The COPD patients treated with ORENCIA developed adverse events more frequently than those treated with placebo (97% vs 88%, respectively). Respiratory disorders occurred more frequently in ORENCIA-treated patients compared to placebo-treated patients (43% vs 24%, respectively) including COPD exacerbation, cough, rhonchi, and dyspnea. A greater percentage of ORENCIA-treated patients developed a serious adverse event compared to placebo-treated patients (27% vs 6%), including COPD exacerbation (3 of 37 patients [8%]) and pneumonia (1 of 37 patients [3%]) [see Warnings and Precautions (5.5)].
Other Adverse Reactions
Adverse events occurring in 3% or more of patients and at least 1% more frequently in ORENCIA-treated patients during placebo-controlled RA studies are summarized in Table 3.
Table 3: Adverse Events Occurring in 3% or More of Patients and at Least 1% More Frequently in ORENCIA-Treated Patients During Placebo-Controlled RA Studies Adverse Event (Preferred Term) ORENCIA
(n=1955)a
PercentagePlacebo
(n=989)b
Percentagea Includes 204 patients on concomitant biologic DMARDs (adalimumab, anakinra, etanercept, or infliximab).
b Includes 134 patients on concomitant biologic DMARDs (adalimumab, anakinra, etanercept, or infliximab).Headache
18
13
Nasopharyngitis
12
9
Dizziness
9
7
Cough
8
7
Back pain
7
6
Hypertension
7
4
Dyspepsia
6
4
Urinary tract infection
6
5
Rash
4
3
Pain in extremity
3
2
Immunogenicity
Antibodies directed against the entire abatacept molecule or to the CTLA-4 portion of abatacept were assessed by ELISA assays in RA patients for up to 2 years following repeated treatment with ORENCIA. Thirty-four of 1993 (1.7%) patients developed binding antibodies to the entire abatacept molecule or to the CTLA-4 portion of abatacept. Because trough levels of abatacept can interfere with assay results, a subset analysis was performed. In this analysis it was observed that 9 of 154 (5.8%) patients that had discontinued treatment with ORENCIA for over 56 days developed antibodies.
Samples with confirmed binding activity to CTLA-4 were assessed for the presence of neutralizing antibodies in a cell-based luciferase reporter assay. Six of 9 (67%) evaluable patients were shown to possess neutralizing antibodies. However, the development of neutralizing antibodies may be underreported due to lack of assay sensitivity.
No correlation of antibody development to clinical response or adverse events was observed.
The data reflect the percentage of patients whose test results were positive for antibodies to abatacept in specific assays. The observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to abatacept with the incidence of antibodies to other products may be misleading.
Clinical Experience in Methotrexate-Naive Patients
Study VI was an active-controlled clinical trial in methotrexate-naive patients [see Clinical Studies (14.1)]. The safety experience in these patients was consistent with Studies I-V.
6.2 Clinical Studies Experience in Adult RA Patients Treated with Subcutaneous ORENCIA
Study SC-1 was a randomized, double-blind, double-dummy, non-inferiority study that compared the efficacy and safety of abatacept administered subcutaneously (SC) and intravenously (IV) in 1457 subjects with rheumatoid arthritis, receiving background methotrexate, and experiencing an inadequate response to methotrexate (MTX-IR) [see Clinical Studies (14.1)]. The safety experience and immunogenicity for ORENCIA administered subcutaneously was consistent with intravenous Studies I-VI. Due to the route of administration, injection site reactions and immunogenicity were evaluated in Study SC-1 and two other smaller studies discussed in the sections below.
Injection Site Reactions in Adult RA Patients Treated with Subcutaneous ORENCIA
Study SC-1 compared the safety of abatacept including injection site reactions following subcutaneous or intravenous administration. The overall frequency of injection site reactions was 2.6% (19/736) and 2.5% (18/721) for the subcutaneous abatacept group and the intravenous abatacept group (subcutaneous placebo), respectively. All these injection site reactions (including hematoma, pruritus, and erythema) were mild (83%) to moderate (17%) in severity, and none necessitated drug discontinuation.
Immunogenicity in Adult RA Patients Treated with Subcutaneous ORENCIA
Study SC-1 compared the immunogenicity to abatacept following subcutaneous or intravenous administration. The overall immunogenicity frequency to abatacept was 1.1% (8/725) and 2.3% (16/710) for the subcutaneous and intravenous groups, respectively. The rate is consistent with previous experience, and there was no correlation of immunogenicity with effects on pharmacokinetics, safety, or efficacy.
Immunogenicity and Safety of Subcutaneous ORENCIA Administration as Monotherapy without an Intravenous Loading Dose
Study SC-2 was conducted to determine the effect of monotherapy use of ORENCIA on immunogenicity following subcutaneous administration without an intravenous load in 100 RA patients, who had not previously received abatacept or other CTLA4Ig, who received either subcutaneous ORENCIA plus methotrexate (n=51) or subcutaneous ORENCIA monotherapy (n=49). No patients in either group developed anti-product antibodies after 4 months of treatment. The safety observed in this study was consistent with that observed in the other subcutaneous studies.
Immunogenicity and Safety of Subcutaneous ORENCIA upon Withdrawal (Three Months) and Restart of Treatment
Study SC-3 in the subcutaneous program was conducted to investigate the effect of withdrawal (three months) and restart of ORENCIA subcutaneous treatment on immunogenicity in RA patients treated concomitantly with methotrexate. One hundred sixty-seven patients were enrolled in the first 3-month treatment period and responders (n=120) were randomized to either subcutaneous ORENCIA or placebo for the second 3-month period (withdrawal period). Patients from this period then received open-label ORENCIA treatment in the final 3-month period of the study (period 3). At the end of the withdrawal period, 0/38 patients who continued to receive subcutaneous ORENCIA developed anti-product antibodies compared to 7/73 (9.6%) of patients who had subcutaneous ORENCIA withdrawn during this period. Half of the patients receiving subcutaneous placebo during the withdrawal period received a single intravenous infusion of ORENCIA at the start of period 3 and half received intravenous placebo. At the end of period 3, when all patients again received subcutaneous ORENCIA, the immunogenicity rates were 1/38 (2.6%) in the group receiving subcutaneous ORENCIA throughout, and 2/73 (2.7%) in the group that had received placebo during the withdrawal period. Upon reinitiating therapy, there were no injection reactions and no differences in response to therapy in patients who were withdrawn from subcutaneous therapy for up to 3 months relative to those who remained on subcutaneous therapy, whether therapy was reintroduced with or without an intravenous loading dose. The safety observed in this study was consistent with that observed in the other studies.
6.3 Clinical Studies Experience in Juvenile Idiopathic Arthritis Patients Treated with Intravenous ORENCIA
In general, the adverse events in pediatric patients were similar in frequency and type to those seen in adult patients [see Warnings and Precautions (5), Adverse Reactions (6)].
Study JIA-1 was a three-part study including an open-label extension that assessed the safety and efficacy of intravenous ORENCIA in 190 pediatric patients, 6 to 17 years of age, with polyarticular juvenile idiopathic arthritis. Overall frequency of adverse events in the 4-month, lead-in, open-label period of the study was 70%; infections occurred at a frequency of 36% [see Clinical Studies (14.2)]. The most common infections were upper respiratory tract infection and nasopharyngitis. The infections resolved without sequelae, and the types of infections were consistent with those commonly seen in outpatient pediatric populations. Other events that occurred at a prevalence of at least 5% were headache, nausea, diarrhea, cough, pyrexia, and abdominal pain.
A total of 6 serious adverse events (acute lymphocytic leukemia, ovarian cyst, varicella infection, disease flare [2], and joint wear) were reported during the initial 4 months of treatment with ORENCIA.
Of the 190 patients with juvenile idiopathic arthritis treated with ORENCIA in clinical trials, there was one case of a hypersensitivity reaction (0.5%). During Periods A, B, and C, acute infusion-related reactions occurred at a frequency of 4%, 2%, and 3%, respectively, and were consistent with the types of events reported in adults.
Upon continued treatment in the open-label extension period, the types of adverse events were similar in frequency and type to those seen in adult patients, except for a single patient diagnosed with multiple sclerosis while on open-label treatment.
Immunogenicity
Antibodies directed against the entire abatacept molecule or to the CTLA-4 portion of abatacept were assessed by ELISA assays in patients with juvenile idiopathic arthritis following repeated treatment with ORENCIA throughout the open-label period. For patients who were withdrawn from therapy for up to 6 months during the double-blind period, the rate of antibody formation to the CTLA-4 portion of the molecule was 41% (22/54), while for those who remained on therapy the rate was 13% (7/54). Twenty of these patients had samples that could be tested for antibodies with neutralizing activity; of these, 8 (40%) patients were shown to possess neutralizing antibodies.
The presence of antibodies was generally transient and titers were low. The presence of antibodies was not associated with adverse events, changes in efficacy, or an effect on serum concentrations of abatacept. For patients who were withdrawn from ORENCIA during the double-blind period for up to 6 months, no serious acute infusion-related events were observed upon re-initiation of ORENCIA therapy.
6.4 Clinical Studies Experience in Juvenile Idiopathic Arthritis Patients Treated with Subcutaneous ORENCIA
Study JIA-2 was an open-label study with a 4-month short-term period and a long-term extension period that assessed the pharmacokinetics (PK), safety, and efficacy of subcutaneous ORENCIA in 205 pediatric patients, 2 to 17 years of age with juvenile idiopathic arthritis. The safety experience and immunogenicity for ORENCIA administered subcutaneously were consistent with the intravenous Study JIA-1.
There were no reported cases of hypersensitivity reactions. Local injection-site reactions occurred at a frequency of 4.4%.
6.5 Clinical Studies Experience in Adult PsA Patients
The safety of ORENCIA was evaluated in 594 patients with psoriatic arthritis (341 patients on ORENCIA and 253 patients on placebo), in two randomized, double-blind, placebo-controlled trials. Of the 341 patients who received ORENCIA, 128 patients received intravenous ORENCIA (PsA-I) and 213 patients received subcutaneous ORENCIA (PsA-II). The safety profile was comparable between studies PsA-I and PsA-II and consistent with the safety profile in rheumatoid arthritis [see Warnings and Precautions (5), Adverse Reactions (6.1, 6.2)].
6.6 Postmarketing Experience
Adverse reactions have been reported during the postapproval use of ORENCIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to ORENCIA. Based on the postmarketing experience in adult RA patients, the following adverse reaction has been identified during postapproval use with ORENCIA.
- Vasculitis (including cutaneous vasculitis and leukocytoclastic vasculitis)
- New or worsening psoriasis
-
7 DRUG INTERACTIONS
7.1 TNF Antagonists
Concurrent administration of a TNF antagonist with ORENCIA has been associated with an increased risk of serious infections and no significant additional efficacy over use of the TNF antagonists alone. Concurrent therapy with ORENCIA and TNF antagonists is not recommended [see Warnings and Precautions (5.1)].
7.2 Other Biologic RA Therapy
There is insufficient experience to assess the safety and efficacy of ORENCIA administered concurrently with other biologic RA therapy, such as anakinra, and therefore such use is not recommended.
7.3 Blood Glucose Testing
Parenteral drug products containing maltose can interfere with the readings of blood glucose monitors that use test strips with glucose dehydrogenase pyrroloquinoline quinone (GDH-PQQ). The GDH-PQQ based glucose monitoring systems may react with the maltose present in ORENCIA for intravenous administration, resulting in falsely elevated blood glucose readings on the day of infusion. When receiving ORENCIA through intravenous administration, patients that require blood glucose monitoring should be advised to consider methods that do not react with maltose, such as those based on glucose dehydrogenase nicotine adenine dinucleotide (GDH-NAD), glucose oxidase, or glucose hexokinase test methods.
ORENCIA for subcutaneous administration does not contain maltose; therefore, patients do not need to alter their glucose monitoring.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ORENCIA during pregnancy. Healthcare professionals are encouraged to register patients and pregnant women are encouraged to enroll themselves by calling 1-877-311-8972.
Risk Summary
The data with ORENCIA use in pregnant women are insufficient to inform on drug-associated risk. In reproductive toxicology studies in rats and rabbits, no fetal malformations were observed with intravenous administration of ORENCIA during organogenesis at doses that produced exposures approximately 29 times the exposure at the maximum recommended human dose (MRHD) of 10 mg/kg/month on an AUC basis. However, in a pre- and postnatal development study in rats, ORENCIA altered immune function in female rats at 11 times the MRHD on an AUC basis.
Data
Human Data
There are no adequate and well-controlled studies of ORENCIA use in pregnant women. The data with ORENCIA use in pregnant women are insufficient to inform on drug-associated risk.
Animal Data
Intravenous administration of abatacept during organogenesis to mice (10, 55, or 300 mg/kg/day), rats (10, 45, or 200 mg/kg/day), and rabbits (10, 45, or 200 mg/kg every 3 days) produced exposures in rats and rabbits that were approximately 29 times the MRHD on an AUC basis (at maternal doses of 200 mg/kg/day in rats and rabbits), and no embryotoxicity or fetal malformations were observed in any species.
In a study of pre- and postnatal development in rats (10, 45, or 200 mg/kg every 3 days from gestation day 6 through lactation day 21), alterations in immune function in female offspring, consisting of a 9-fold increase in T-cell-dependent antibody response relative to controls on postnatal day (PND) 56 and thyroiditis in a single female pup on PND 112, occurred at approximately 11 times the MRHD on an AUC basis (at a maternal dose of 200 mg/kg). No adverse effects were observed at approximately 3 times the MRHD (a maternal dose of 45 mg/kg). It is not known if immunologic perturbations in rats are relevant indicators of a risk for development of autoimmune diseases in humans exposed in utero to abatacept. Exposure to abatacept in the juvenile rat, which may be more representative of the fetal immune system state in the human, resulted in immune system abnormalities including inflammation of the thyroid and pancreas [see Nonclinical Toxicology (13.2)].
8.4 Pediatric Use
In Study JIA-1, ORENCIA with intravenous administration was shown to reduce signs and symptoms of active polyarticular JIA in patients 6 to 17 years of age [see Clinical Studies (14.2)]. ORENCIA with intravenous administration has not been studied in patients younger than 6 years of age.
In Study JIA-2, the PK and safety of ORENCIA prefilled syringe for subcutaneous injection have been studied in patients 2 to 17 years of age. The efficacy of ORENCIA for subcutaneous injection in children 2 to 17 years of age is based on pharmacokinetic exposure and extrapolation of established efficacy of intravenous ORENCIA in polyarticular JIA patients and subcutaneous ORENCIA in patients with RA [see Clinical Pharmacology (12.3) and Clinical Studies (14.2)]. The safety and immunogenicity of ORENCIA for subcutaneous injection in children 2 to 17 years of age were assessed descriptively [see Adverse Reactions (6.4)]. ORENCIA may be used as monotherapy or concomitantly with methotrexate.
The safety and efficacy of ORENCIA ClickJect autoinjector for subcutaneous injection have not been studied in patients under 18 years of age.
Studies in juvenile rats exposed to ORENCIA prior to immune system maturity have shown immune system abnormalities including an increase in the incidence of infections leading to death as well as inflammation of the thyroid and pancreas [see Nonclinical Toxicology (13.2)]. Studies in adult mice and monkeys have not demonstrated similar findings. As the immune system of the rat is undeveloped in the first few weeks after birth, the relevance of these results to humans is unknown.
The safety and efficacy of ORENCIA in pediatric patients for uses other than juvenile idiopathic arthritis have not been established.
It is unknown if abatacept can cross the placenta into the fetus when the woman is treated with abatacept during pregnancy. Since abatacept is an immunomodulatory agent, the safety of administering live vaccines in infants exposed in utero to abatacept is unknown. Risk and benefits should be considered prior to vaccinating such infants.
8.5 Geriatric Use
A total of 323 patients 65 years of age and older, including 53 patients 75 years and older, received ORENCIA in clinical studies. No overall differences in safety or effectiveness were observed between these patients and younger patients, but these numbers are too low to rule out differences. The frequency of serious infection and malignancy among ORENCIA-treated patients over age 65 was higher than for those under age 65. Because there is a higher incidence of infections and malignancies in the elderly population in general, caution should be used when treating the elderly.
- 10 OVERDOSAGE
-
11 DESCRIPTION
ORENCIA® (abatacept) is a selective T cell costimulation modulator. ORENCIA is a soluble fusion protein that consists of the extracellular domain of human cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) linked to the modified Fc (hinge, CH2, and CH3 domains) portion of human immunoglobulin G1 (IgG1). Abatacept is produced by recombinant DNA technology in a mammalian cell expression system. The apparent molecular weight of abatacept is 92 kilodaltons.
ORENCIA for Injection is a lyophilized powder for intravenous infusion. ORENCIA for Injection is supplied as a sterile, white, preservative-free, lyophilized powder for reconstitution and dilution prior to intravenous administration. Following reconstitution of the lyophilized powder with 10 mL of Sterile Water for Injection, USP, the solution of ORENCIA is clear, colorless to pale yellow, with a pH range of 7.2 to 7.8. Each single-use vial of ORENCIA for Injection provides 250 mg abatacept, maltose (500 mg), monobasic sodium phosphate (17.2 mg), and sodium chloride (14.6 mg) for administration.
ORENCIA Injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale-yellow solution with a pH range of 6.8 to 7.4 for subcutaneous administration. ORENCIA Injection is supplied as a single-dose prefilled syringe or as a single-dose ClickJect autoinjector (see Table 4).
Table 4: Contents of ORENCIA Subcutaneous Injection Presentation Active Ingredient Quantity and Label Volume Inactive Ingredient Content ORENCIA Injection 50 mg/0.4 mL Prefilled Syringe
50 mg of abatacept in
0.4 mL of solutiondibasic sodium phosphate anhydrous (0.335 mg)
monobasic sodium phosphate monohydrate (0.114 mg)
poloxamer 188 (3.2 mg)
sucrose (68 mg)
qs to 0.4 mL water for injection
ORENCIA Injection 87.5 mg/0.7 mL Prefilled Syringe
87.5 mg of abatacept in
0.7 mL of solutiondibasic sodium phosphate anhydrous (0.587 mg)
monobasic sodium phosphate monohydrate (0.200 mg)
poloxamer 188 (5.6 mg)
sucrose (119 mg)
qs to 0.7 mL water for injection
ORENCIA Injection 125 mg/mL Prefilled Syringe and ClickJect Autoinjector
125 mg of abatacept in
1 mL of solutiondibasic sodium phosphate anhydrous (0.838 mg)
monobasic sodium phosphate monohydrate (0.286 mg)
poloxamer 188 (8 mg)
sucrose (170 mg)
qs to 1.0 mL water for injection
Unlike the lyophilized formulation for intravenous use, the ORENCIA solutions for subcutaneous administration contain no maltose.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Abatacept, a selective costimulation modulator, inhibits T cell (T lymphocyte) activation by binding to CD80 and CD86, thereby blocking interaction with CD28. This interaction provides a costimulatory signal necessary for full activation of T lymphocytes. Activated T lymphocytes are implicated in the pathogenesis of RA and PsA and are found in the synovium of patients with RA and PsA.
In vitro, abatacept decreases T cell proliferation and inhibits the production of the cytokines TNF alpha (TNFα), interferon-γ, and interleukin-2. In a rat collagen-induced arthritis model, abatacept suppresses inflammation, decreases anti-collagen antibody production, and reduces antigen specific production of interferon-γ. The relationship of these biological response markers to the mechanisms by which ORENCIA exerts its clinical effects is unknown.
12.2 Pharmacodynamics
In clinical trials with ORENCIA at doses approximating 10 mg/kg, decreases were observed in serum levels of soluble interleukin-2 receptor (sIL-2R), interleukin-6 (IL-6), rheumatoid factor (RF), C-reactive protein (CRP), matrix metalloproteinase-3 (MMP3), and TNFα. The relationship of these biological response markers to the mechanisms by which ORENCIA exerts its clinical effects is unknown.
12.3 Pharmacokinetics
Healthy Adults and Adult RA - Intravenous Administration
The pharmacokinetics of abatacept were studied in healthy adult subjects after a single 10 mg/kg intravenous infusion and in RA patients after multiple 10 mg/kg intravenous infusions (see Table 5).
Table 5: Pharmacokinetic Parameters (Mean, Range) in Healthy Subjects and RA Patients After 10 mg/kg Intravenous Infusion(s) PK Parameter Healthy Subjects
(After 10 mg/kg Single Dose)
n=13RA Patients
(After 10 mg/kg Multiple Dosesa)
n=14a Multiple intravenous infusions were administered at days 1, 15, 30, and monthly thereafter. Peak Concentration (Cmax) [mcg/mL]
292 (175-427)
295 (171-398)
Terminal half-life (t1/2) [days]
16.7 (12-23)
13.1 (8-25)
Systemic clearance (CL) [mL/h/kg]
0.23 (0.16-0.30)
0.22 (0.13-0.47)
Volume of distribution (Vss) [L/kg]
0.09 (0.06-0.13)
0.07 (0.02-0.13)
The pharmacokinetics of abatacept in RA patients and healthy subjects appeared to be comparable. In RA patients, after multiple intravenous infusions, the pharmacokinetics of abatacept showed proportional increases of Cmax and AUC over the dose range of 2 mg/kg to 10 mg/kg. At 10 mg/kg, serum concentration appeared to reach a steady state by day 60 with a mean (range) trough concentration of 24 mcg/mL (1 to 66 mcg/mL). No systemic accumulation of abatacept occurred upon continued repeated treatment with 10 mg/kg at monthly intervals in RA patients.
Population pharmacokinetic analyses in RA patients revealed that there was a trend toward higher clearance of abatacept with increasing body weight. Age and gender (when corrected for body weight) did not affect clearance. Concomitant methotrexate, NSAIDs, corticosteroids, and TNF blocking agents did not influence abatacept clearance.
No formal studies were conducted to examine the effects of either renal or hepatic impairment on the pharmacokinetics of abatacept.
Adult RA - Subcutaneous Administration
Abatacept exhibited linear pharmacokinetics following subcutaneous administration. The mean (range) for Cmin and Cmax at steady state observed after 85 days of treatment was 32.5 mcg/mL (6.6 to 113.8 mcg/mL) and 48.1 mcg/mL (9.8 to 132.4 mcg/mL), respectively. The bioavailability of abatacept following subcutaneous administration relative to intravenous administration is 78.6%. Mean estimates for systemic clearance (0.28 mL/h/kg), volume of distribution (0.11 L/kg), and terminal half-life (14.3 days) were comparable between subcutaneous and intravenous administration.
Study SC-2 was conducted to determine the effect of monotherapy use of ORENCIA on immunogenicity following subcutaneous administration without an intravenous load. When the intravenous loading dose was not administered, a mean trough concentration of 12.6 mcg/mL was achieved after 2 weeks of dosing.
Consistent with the intravenous data, population pharmacokinetic analyses for subcutaneous abatacept in RA patients revealed that there was a trend toward higher clearance of abatacept with increasing body weight. Age and gender (when corrected for body weight) did not affect apparent clearance. Concomitant medication, such as methotrexate, corticosteroids, and NSAIDs, did not influence abatacept apparent clearance.
Juvenile Idiopathic Arthritis - Intravenous Administration
In Study JIA-1 among patients 6 to 17 years of age, the mean (range) steady state serum peak and trough concentrations of abatacept were 217 mcg/mL (57 to 700 mcg/mL) and 11.9 mcg/mL (0.15 to 44.6 mcg/mL). Population pharmacokinetic analyses of the serum concentration data showed that clearance of abatacept increased with baseline body weight. The estimated mean (range) clearance of abatacept in the juvenile idiopathic arthritis patients was 0.4 mL/h/kg (0.20 to 1.12 mL/h/kg). After accounting for the effect of body weight, the clearance of abatacept was not related to age and gender. Concomitant methotrexate, corticosteroids, and NSAIDs were also shown not to influence abatacept clearance.
Juvenile Idiopathic Arthritis - Subcutaneous Administration
In Study JIA-2 among patients 2 to 17 years of age, steady state of abatacept was achieved by Day 85 following the weekly body-weight–tiered subcutaneous abatacept dosing. Comparable trough concentrations across weight tiers and age groups were achieved by the body-weight–tiered subcutaneous dosing regimen. The mean (range) trough concentration of abatacept at Day 113 was 44.4 mcg/mL (13.4 to 88.1 mcg/mL), 46.6 mcg/mL (22.4 to 97.0 mcg/mL), and 38.5 mcg/mL (9.3 to 73.2 mcg/mL) in pediatric JIA patients weighing 10 to <25 kg, 25 to <50 kg, and ≥50 kg, respectively.
Consistent with the intravenous data, population pharmacokinetic analyses for subcutaneous abatacept in JIA patients revealed that there was a trend toward higher clearance of abatacept with increasing body weight. Age and gender (when corrected for body weight) did not affect apparent clearance. Concomitant medication, such as methotrexate, corticosteroids, and NSAIDs, did not influence abatacept apparent clearance.
Adult Psoriatic Arthritis - Intravenous and Subcutaneous Administration
In Study PsA-I, a dose ranging study, IV abatacept was administered at 3 mg/kg, 10 mg/kg (weight range-based dosing: 500 mg for patients weighing less than 60 kg, 750 mg for patients weighing 60 to 100 kg, and 1000 mg for patients weighing greater than 100 kg), or two doses of 30 mg/kg followed by weight range-based dose of 10 mg/kg. Following monthly IV administration, abatacept showed linear PK over the dose range of 3 mg/kg to 10 mg/kg. At 10 mg/kg, the steady state of abatacept was reached by Day 57 and the geometric mean (CV%) trough concentration (Cmin) was 24.3 mcg/mL (40.8%) at Day 169. In Study PsA-II following weekly SC administration of abatacept at 125 mg, the steady state of abatacept was reached at Day 57 and the geometric mean (CV%) Cmin was 25.6 mcg/mL (47.7%) at Day 169.
Consistent with the RA results, population pharmacokinetic analyses for abatacept in psoriatic arthritis patients revealed that there was a trend toward higher clearance (L/h) of abatacept with increasing body weight. In addition, relative to the RA patients with the same body weight, abatacept clearance in psoriatic arthritis patients was approximately 8% lower, resulting in higher abatacept exposures in patients with PsA. This slight difference in exposures, however, is not considered to be clinically meaningful.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a mouse carcinogenicity study, weekly subcutaneous injections of 20, 65, or 200 mg/kg of abatacept administered for up to 84 weeks in males and 88 weeks in females were associated with increases in the incidence of malignant lymphomas (all doses) and mammary gland tumors (intermediate- and high-dose in females). The mice from this study were infected with murine leukemia virus and mouse mammary tumor virus. These viruses are associated with an increased incidence of lymphomas and mammary gland tumors, respectively, in immunosuppressed mice. The doses used in these studies produced exposures 0.8, 2.0, and 3.0 times higher, respectively, than the exposure associated with the maximum recommended human dose (MRHD) of 10 mg/kg based on AUC (area under the time-concentration curve). The relevance of these findings to the clinical use of ORENCIA is unknown.
In a one-year toxicity study in cynomolgus monkeys, abatacept was administered intravenously once weekly at doses up to 50 mg/kg (producing 9 times the MRHD exposure based on AUC). Abatacept was not associated with any significant drug-related toxicity. Reversible pharmacological effects consisted of minimal transient decreases in serum IgG and minimal to severe lymphoid depletion of germinal centers in the spleen and/or lymph nodes. No evidence of lymphomas or preneoplastic morphologic changes was observed, despite the presence of a virus (lymphocryptovirus) known to cause these lesions in immunosuppressed monkeys within the time frame of this study. The relevance of these findings to the clinical use of ORENCIA is unknown.
No mutagenic potential of abatacept was observed in the in vitro bacterial reverse mutation (Ames) or Chinese hamster ovary/hypoxanthine guanine phosphoribosyl-transferase (CHO/HGPRT) forward point mutation assays with or without metabolic activation, and no chromosomal aberrations were observed in human lymphocytes treated with abatacept with or without metabolic activation.
Abatacept had no adverse effects on male or female fertility in rats at doses up to 200 mg/kg every three days (11 times the MRHD exposure based on AUC).
13.2 Animal Toxicology and/or Pharmacology
A juvenile animal study was conducted in rats dosed with abatacept from 4 to 94 days of age in which an increase in the incidence of infections leading to death occurred at all doses compared with controls. Altered T-cell subsets including increased T-helper cells and reduced T-regulatory cells were observed. In addition, inhibition of T-cell-dependent antibody responses (TDAR) was observed. Upon following these animals into adulthood, lymphocytic inflammation of the thyroid and pancreatic islets was observed.
In studies of adult mice and monkeys, inhibition of TDAR was apparent. However, infection and mortality, altered T-helper cells, and inflammation of thyroid and pancreas were not observed.
-
14 CLINICAL STUDIES
14.1 Adult Rheumatoid Arthritis
The efficacy and safety of ORENCIA for intravenous administration were assessed in six randomized, double-blind, controlled studies (five placebo-controlled and one active-controlled) in patients ≥18 years of age with active RA diagnosed according to American College of Rheumatology (ACR) criteria. Studies I, II, III, IV, and VI required patients to have at least 12 tender and 10 swollen joints at randomization. Study V did not require any specific number of tender or swollen joints. ORENCIA or placebo treatment was given intravenously at weeks 0, 2, and 4 and then every 4 weeks thereafter in intravenous Studies I, II, III, IV, and VI. The safety and efficacy of ORENCIA for subcutaneous administration were assessed in Study SC-1, which was a randomized, double-blind, double-dummy, non-inferiority study that compared abatacept administered subcutaneously and intravenously in 1457 subjects with rheumatoid arthritis (RA), receiving background methotrexate (MTX), and experiencing an inadequate response to methotrexate (MTX-IR).
Study I evaluated ORENCIA as monotherapy in 122 patients with active RA who had failed at least one non-biologic DMARD or etanercept. In Study II and Study III, the efficacy and safety of ORENCIA were assessed in patients with an inadequate response to methotrexate and who were continued on their stable dose of methotrexate. In Study IV, the efficacy and safety of ORENCIA were assessed in patients with an inadequate response to a TNF blocking agent, with the TNF blocking agent discontinued prior to randomization; other DMARDs were permitted. Study V primarily assessed safety in patients with active RA requiring additional intervention in spite of current therapy with DMARDs; all DMARDs used at enrollment were continued. Patients in Study V were not excluded for comorbid medical conditions. In Study VI, the efficacy and safety of ORENCIA were assessed in methotrexate-naive patients with RA of less than 2 years disease duration. In Study VI, patients previously naive to methotrexate were randomized to receive ORENCIA plus methotrexate or methotrexate plus placebo. In Study SC-1, the goal was to demonstrate the efficacy and safety of ORENCIA subcutaneous relative to ORENCIA intravenous administration in subjects with moderate to severely active RA and experiencing inadequate response to methotrexate, using a non-inferiority study design.
Study I patients were randomized to receive one of three doses of ORENCIA (0.5, 2, or 10 mg/kg) or placebo ending at week 8. Study II patients were randomized to receive ORENCIA 2 or 10 mg/kg or placebo for 12 months. Study III, IV, V, and VI patients were randomized to receive a dose of ORENCIA based on weight range or placebo for 12 months (Studies III, V, and VI) or 6 months (Study IV). The dose of ORENCIA was 500 mg for patients weighing less than 60 kg, 750 mg for patients weighing 60 to 100 kg, and 1000 mg for patients weighing greater than 100 kg. In Study SC-1, patients were randomized with stratification by body weight (<60 kg, 60 to 100 kg, >100 kg) to receive ORENCIA 125 mg subcutaneous injections weekly, after a single intravenous loading dose of ORENCIA based on body weight or ORENCIA intravenously on Days 1, 15, 29, and every four weeks thereafter. Subjects continued taking their current dose of methotrexate from the day of randomization.
Clinical Response
The percent of ORENCIA-treated patients achieving ACR 20, 50, and 70 responses and major clinical response in Studies I, III, IV, and VI are shown in Table 6. ORENCIA-treated patients had higher ACR 20, 50, and 70 response rates at 6 months compared to placebo-treated patients. Month 6 ACR response rates in Study II for the 10 mg/kg group were similar to the ORENCIA group in Study III.
In Studies III and IV, improvement in the ACR 20 response rate versus placebo was observed within 15 days in some patients and within 29 days versus methotrexate in Study VI. In Studies II, III, and VI, ACR response rates were maintained to 12 months in ORENCIA-treated patients. ACR responses were maintained up to three years in the open-label extension of Study II. In Study III, ORENCIA-treated patients experienced greater improvement than placebo-treated patients in morning stiffness.
In Study VI, a greater proportion of patients treated with ORENCIA plus methotrexate achieved a low level of disease activity as measured by a DAS28-CRP less than 2.6 at 12 months compared to those treated with methotrexate plus placebo (Table 6). Of patients treated with ORENCIA plus methotrexate who achieved DAS28-CRP less than 2.6, 54% had no active joints, 17% had one active joint, 7% had two active joints, and 22% had three or more active joints, where an active joint was a joint that was rated as tender or swollen or both.
In Study SC-1, the main outcome measure was ACR 20 at 6 months. The pre-specified non-inferiority margin was a treatment difference of −7.5%. As shown in Table 6, the study demonstrated non-inferiority of ORENCIA administered subcutaneously to intravenous infusions of ORENCIA with respect to ACR 20 responses up to 6 months of treatment. ACR 50 and 70 responses are also shown in Table 6. No major differences in ACR responses were observed between intravenous and subcutaneous treatment groups in subgroups based on weight categories (less than 60 kg, 60 to 100 kg, and more than 100 kg; data not shown).
Table 6: Clinical Responses in Controlled Trials Percent of Patients Intravenous Administration Subcutaneous Administration Inadequate Response to
DMARDsInadequate
Response to
Methotrexate
(MTX)Inadequate Response to
TNF Blocking AgentMTX-Naive Inadequate
Response to MTXStudy I Study III Study IV Study VI Study SC-1 * p<0.05, ORENCIA (ORN) vs placebo (PBO) or MTX.
† p<0.01, ORENCIA vs placebo or MTX.
‡ p<0.001, ORENCIA vs placebo or MTX.
§ 95% CI: −4.2, 4.8 (based on prespecified margin for non-inferiority of −7.5%).
a 10 mg/kg.
b Dosing based on weight range [see Dosage and Administration (2.1)].
c Major clinical response is defined as achieving an ACR 70 response for a continuous 6-month period.
d Refer to text for additional description of remaining joint activity.
e Per protocol data is presented in table. For ITT; n=736, 721 for SC and IV ORENCIA, respectively.Response Rate
ORNa
n=32PBO
n=32ORNb
+MTX
n=424PBO
+MTX
n=214ORNb +
DMARDs
n=256PBO +
DMARDs
n=133ORNb
+MTX
n=256PBO
+MTX
n=253ORNe
SC
+MTX
n=693ORNe
IV
+MTX
n=678ACR 20
Month 3
53%
31%
62%‡
37%
46%‡
18%
64%*
53%
68%
69%
Month 6
NA
NA
68%‡
40%
50%‡
20%
75%†
62%
76%§
76%
Month 12
NA
NA
73%‡
40%
NA
NA
76%‡
62%
NA
NA
ACR 50
Month 3
16%
6%
32%‡
8%
18%†
6%
40%‡
23%
33%
39%
Month 6
NA
NA
40%‡
17%
20%‡
4%
53%‡
38%
52%
50%
Month 12
NA
NA
48%‡
18%
NA
NA
57%‡
42%
NA
NA
ACR 70
Month 3
6%
0
13%‡
3%
6%*
1%
19%†
10%
13%
16%
Month 6
NA
NA
20%‡
7%
10%†
2%
32%†
20%
26%
25%
Month 12
NA
NA
29%‡
6%
NA
NA
43%‡
27%
NA
NA
Major
Clinical
ResponsecNA
NA
14%‡
2%
NA
NA
27%‡
12%
NA
NA
DAS28-CRP
<2.6dMonth 12
NA
NA
NA
NA
NA
NA
41%‡
23%
NA
NA
The results of the components of the ACR response criteria for Studies III, IV, and SC-1 are shown in Table 7 (results at Baseline [BL] and 6 months [6 M]). In ORENCIA-treated patients, greater improvement was seen in all ACR response criteria components through 6 and 12 months than in placebo-treated patients.
Table 7: Components of ACR Responses at 6 Months Intravenous Administration Subcutaneous Administration Inadequate Response to
Methotrexate (MTX)Inadequate Response to
TNF Blocking AgentInadequate Response to
MTXStudy III Study IV Study SC-1c † p<0.01, ORENCIA (ORN) vs placebo (PBO), based on mean percent change from baseline.
‡ p<0.001, ORENCIA vs placebo, based on mean percent change from baseline.
a Visual analog scale: 0 = best, 100 = worst.
b Health Assessment Questionnaire: 0 = best, 3 = worst; 20 questions; 8 categories: dressing and grooming, arising, eating,
walking, hygiene, reach, grip, and activities.
c SC-1 is a non-inferiority study. Per protocol data is presented in table.ORN
+MTX
n=424PBO
+MTX
n=214ORN
+DMARDs
n=256PBO
+DMARDs
n=133ORN SC
+MTX
n=693ORN IV
+MTX
n=678Component
(median)BL
6 M
BL
6 M
BL
6 M
BL
6 M
BL
6 M
BL
6 M
Number of
tender joints
(0-68)28
7‡
31
14
30
13‡
31
24
27
5
27
6
Number of
swollen joints
(0-66)19
5‡
20
11
21
10‡
20
14
18
4
18
3
Paina
67
27‡
70
50
73
43†
74
64
71
25
70
28
Patient global
assessmenta66
29‡
64
48
71
44‡
73
63
70
26
68
27
Disability
indexb1.75
1.13‡
1.75
1.38
1.88
1.38‡
2.00
1.75
1.88
1.00
1.75
1.00
Physician
global assessmenta69
21‡
68
40
71
32‡
69
54
65
16
65
15
CRP (mg/dL)
2.2
0.9‡
2.1
1.8
3.4
1.3‡
2.8
2.3
1.6
0.7
1.8
0.7
The percent of patients achieving the ACR 50 response for Study III by visit is shown in Figure 1. The time course for the ORENCIA group in Study VI was similar to that in Study III.
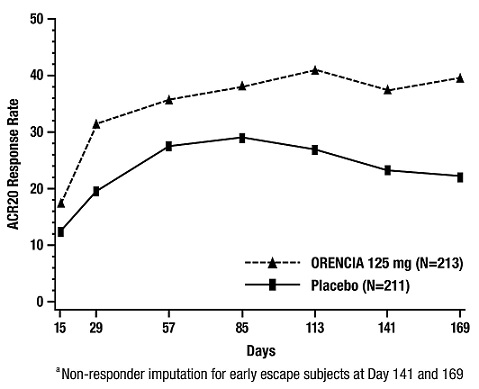
Figure 1: Percent of Patients Achieving ACR 50 Response by Visit* (Study III)
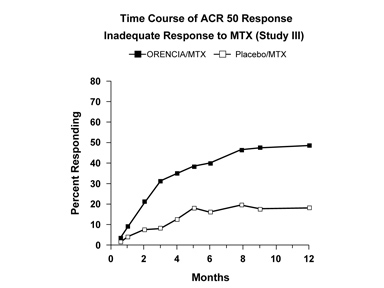
*The same patients may not have responded at each time point.The percent of patients achieving the ACR 50 response for Study SC-1 in the ORENCIA subcutaneous (SC) and intravenous (IV) treatment arms at each treatment visit was as follows: Day 15—SC 3%, IV 5%; Day 29—SC 11%, IV 14%; Day 57—SC 24%, IV 30%; Day 85—SC 33%, IV 38%; Day 113—SC 39%, IV 41%; Day 141—SC 46%, IV 47%; Day 169—SC 51%, IV 50%.
Radiographic Response
In Study III and Study VI, structural joint damage was assessed radiographically and expressed as change from baseline in the Genant-modified Total Sharp Score (TSS) and its components, the Erosion Score (ES) and Joint Space Narrowing (JSN) score. ORENCIA/methotrexate slowed the progression of structural damage compared to placebo/methotrexate after 12 months of treatment as shown in Table 8.
Table 8: Mean Radiographic Changes in Study IIIa and Study VIb Parameter ORENCIA/MTX Placebo/MTX Differences P-valued a Patients with an inadequate response to MTX.
b MTX-naive patients.
c Patients received 1 year of placebo/MTX followed by 1 year of ORENCIA/MTX.
d Based on a nonparametric ANCOVA model.Study III
First Year
TSS
1.07
2.43
1.36
<0.01
ES
0.61
1.47
0.86
<0.01
JSN score
0.46
0.97
0.51
<0.01
Second Year
TSS
0.48
0.74c
-
-
ES
0.23
0.22c
-
-
JSN score
0.25
0.51c
-
-
Study VI
First Year
TSS
0.6
1.1
0.5
0.04
In the open-label extension of Study III, 75% of patients initially randomized to ORENCIA/methotrexate and 65% of patients initially randomized to placebo/methotrexate were evaluated radiographically at Year 2. As shown in Table 8, progression of structural damage in ORENCIA/methotrexate-treated patients was further reduced in the second year of treatment.
Following 2 years of treatment with ORENCIA/methotrexate, 51% of patients had no progression of structural damage as defined by a change in the TSS of zero or less compared with baseline. Fifty-six percent (56%) of ORENCIA/methotrexate-treated patients had no progression during the first year compared to 45% of placebo/methotrexate-treated patients. In their second year of treatment with ORENCIA/methotrexate, more patients had no progression than in the first year (65% vs 56%).
Physical Function Response and Health-Related Outcomes
Improvement in physical function was measured by the Health Assessment Questionnaire Disability Index (HAQ-DI). In the HAQ-DI, ORENCIA demonstrated greater improvement from baseline versus placebo in Studies II-V and versus methotrexate in Study VI. In Study SC-1, improvement from baseline as measured by HAQ-DI at 6 months and over time was similar between subcutaneous and intravenous administration. The results from Studies II and III are shown in Table 9. Similar results were observed in Study V compared to placebo and in Study VI compared to methotrexate. During the open-label period of Study II, the improvement in physical function has been maintained for up to 3 years.
Table 9: Mean Improvement from Baseline in Health Assessment Questionnaire Disability Index (HAQ-DI) Inadequate Response to Methotrexate Study II Study III *** p<0.001, ORENCIA vs placebo.
a 10 mg/kg.
b Dosing based on weight range [see Dosage and Administration (2.1)].
c Modified Health Assessment Questionnaire: 0 = best, 3 = worst; 8 questions; 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities.
d Health Assessment Questionnaire: 0 = best, 3 = worst; 20 questions; 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities.
HAQ Disability IndexORENCIAa
+MTX
(n=115)Placebo
+MTX
(n=119)ORENCIAb
+MTX
(n=422)Placebo
+MTX
(n=212)Baseline (Mean)
0.98c
0.97c
1.69d
1.69d
Mean Improvement
Year 1
0.40c,***
0.15c
0.66d,***
0.37dHealth-related quality of life was assessed by the SF-36 questionnaire at 6 months in Studies II, III, and IV and at 12 months in Studies II and III. In these studies, improvement was observed in the ORENCIA group as compared with the placebo group in all 8 domains of the SF-36 as well as the Physical Component Summary (PCS) and the Mental Component Summary (MCS).
14.2 Juvenile Idiopathic Arthritis
Juvenile Idiopathic Arthritis - Intravenous Administration
The safety and efficacy of ORENCIA with intravenous administration were assessed in Study JIA-1, a three-part study including an open-label extension in children with polyarticular juvenile idiopathic arthritis (JIA). Patients 6 to 17 years of age (n=190) with moderately to severely active polyarticular JIA who had an inadequate response to one or more DMARDs, such as methotrexate or TNF antagonists, were treated. Patients had a disease duration of approximately 4 years with moderately to severely active disease at study entry, as determined by baseline counts of active joints (mean, 16) and joints with loss of motion (mean, 16); patients had elevated C-reactive protein (CRP) levels (mean, 3.2 mg/dL) and ESR (mean, 32 mm/h). The patients enrolled had subtypes of JIA that at disease onset included Oligoarticular (16%), Polyarticular (64%; 20% were rheumatoid factor positive), and Systemic (20%). At study entry, 74% of patients were receiving methotrexate (mean dose, 13.2 mg/m2 per week) and remained on a stable dose of methotrexate (those not receiving methotrexate did not initiate methotrexate treatment during the study).
In Period A (open-label, lead-in), patients received 10 mg/kg (maximum 1000 mg per dose) intravenously on days 1, 15, 29, and monthly thereafter. Response was assessed utilizing the ACR Pediatric 30 definition of improvement, defined as ≥30% improvement in at least 3 of the 6 JIA core set variables and ≥30% worsening in not more than 1 of the 6 JIA core set variables. Patients demonstrating an ACR Pedi 30 response at the end of Period A were randomized into the double-blind phase (Period B) and received either ORENCIA or placebo for 6 months or until disease flare. Disease flare was defined as a ≥30% worsening in at least 3 of the 6 JIA core set variables with ≥30% improvement in not more than 1 of the 6 JIA core set variables; ≥2 cm of worsening of the Physician or Parent Global Assessment was necessary if used as 1 of the 3 JIA core set variables used to define flare, and worsening in ≥2 joints was necessary if the number of active joints or joints with limitation of motion was used as 1 of the 3 JIA core set variables used to define flare.
At the conclusion of Period A, pediatric ACR 30/50/70 responses were 65%, 50%, and 28%, respectively. Pediatric ACR 30 responses were similar in all subtypes of JIA studied.
During the double-blind randomized withdrawal phase (Period B), ORENCIA-treated patients experienced significantly fewer disease flares compared to placebo-treated patients (20% vs 53%); 95% CI of the difference (15%, 52%). The risk of disease flare among patients continuing on ORENCIA was less than one-third than that for patients withdrawn from ORENCIA treatment (hazard ratio=0.31, 95% CI [0.16, 0.59]). Among patients who received ORENCIA throughout the study (Period A, Period B, and the open-label extension Period C), the proportion of pediatric ACR 30/50/70 responders has remained consistent for 1 year.
Juvenile Idiopathic Arthritis - Subcutaneous Administration
ORENCIA for subcutaneous administration without an intravenous loading dose was assessed in Study JIA-2, a 2-period, open-label study that included children 2 to 17 years of age (n=205). Patients had active polyarticular disease at the time of the study and had inadequate response to at least one nonbiologic or biologic DMARD. The patient subtypes at study entry included Polyarticular (79%; 22% were rheumatoid factor positive), Extended and Persistent Oligoarticular (14%), Enthesitis-Related Arthritis (1%), and Systemic (2%). Patients had a mean disease duration of 2.5 years with active joints (mean, 11.9), joints with loss of motion (mean, 10.4), and elevated C-reactive protein (CRP) levels (mean, 1.2 mg/dL). At study entry, 80% of patients were receiving methotrexate and remained on a stable dose of methotrexate. Patients received weekly open-label ORENCIA subcutaneously by a weight-tiered dosing regimen. The primary objective of the study was evaluation of PK in order to support the extrapolation of efficacy based on exposure to ORENCIA supported by descriptive efficacy [see Clinical Pharmacology (12.3)].
JIA ACR 30/50/70 responses assessed at 4 months in the 2- to 17-year-old patients were consistent with the results from the intravenous study, JIA-1.
14.3 Adult Psoriatic Arthritis
The efficacy of ORENCIA was assessed in 594 patients with psoriatic arthritis, in two randomized, double-blind, placebo-controlled studies (Studies PsA-I and PsA-II) in adult patients, age 18 years and older. Patients had active psoriatic arthritis (≥3 swollen joints and ≥3 tender joints) despite prior treatment with DMARD therapy and had one qualifying psoriatic skin lesion of at least 2 cm in diameter. In PsA-I and PsA-II, 37% and 61% of patients, respectively, were treated with TNFi previously.
In PsA-I, a dose-ranging study, 170 patients received study drug IV at Day 1, 15, 29, and then every 28 days thereafter in a double blind manner for 24 weeks, followed by open-label ORENCIA every 28 days. Patients were randomized to receive placebo or ORENCIA 3 mg/kg, 10 mg/kg (weight range-based dosing: 500 mg for patients weighing less than 60 kg, 750 mg for patients weighing 60 to 100 kg, and 1000 mg for patients weighing greater than 100 kg), or two doses of 30 mg/kg followed by weight range-based dosing of 10 mg/kg without escape for 24 weeks. Patients were allowed to receive stable doses of concomitant methotrexate, low dose corticosteroids (equivalent to ≤10 mg of prednisone) and/or NSAIDs during the trial. At enrollment, approximately 60% of patients were receiving methotrexate. At baseline, the mean (SD) CRP for ORENCIA IV was 17 mg/L (33.0) and mean number (SD) of tender joints and swollen joints was 22.2 (14.3) and 10.9 (7.6), respectively.
In PsA-II, 424 patients were randomized 1:1 to receive weekly doses of SC placebo or ORENCIA 125 mg without a loading dose for 24 weeks-in a double-blind manner, followed by open-label ORENCIA 125 mg SC weekly. Patients were allowed to receive stable doses of concomitant methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, low dose corticosteroids (equivalent to ≤10 mg of prednisone) and/or NSAIDs during the trial. At randomization, 60.4% of patients were receiving methotrexate. The baseline disease characteristics included presence of joint erosion on X-rays in 84% (341/424) with a mean (SD) PsA-modified Sharp van der Heijde erosion score (SHS) of 10.8 (24.2), elevated serum C reactive protein (CRP) in 66% [277/424]) with a mean (SD) of 14.1 mg/L (25.9), and polyarticular disease in 98% (416/424) of patients with a mean number (SD) of tender joints and swollen joints of 20.2 (13.3) and 11.6 (7.5), respectively. Patients who had not achieved at least a 20% improvement from baseline in their swollen and tender joint counts by Week 16 escaped to open-label ORENCIA 125 mg SC weekly.
The primary endpoint for both PsA-I and PsA-II was the proportion of patients achieving ACR 20 response at Week 24 (Day 169).
Clinical Response
A higher proportion of patients achieved an ACR20 response after treatment with ORENCIA 10 mg/kg IV (weight range-based dosing as described above) or 125 mg SC compared to placebo at Week 24. Responses were seen regardless of prior TNFi treatment and regardless of concomitant non-biologic DMARD treatment. The percent of patients achieving ACR 20, 50, or 70 responses in Studies PsA-I and PsA-II are presented in Table 10 below.
Table 10: Proportion of Patients With ACR Responses at Week 24 in Studies PsA-I and PsA-IIa PsA-I PsA-II * p<0.05 versus placebo
a Patients who had less than 20% improvement in tender or swollen joint counts at Week 16 met escape criteria and were considered non-responders.
b Weight range-based dosing (as described above).ORENCIA
10mg/kg IVb
N=40
Placebo
N=42ORENCIA 125 mg
SC
N=213
Placebo
N=211ACR 20
47.5%*
19.0%
39.4%*
22.3%
ACR 50
25.0%
2.4%
19.2%
12.3%
ACR 70
12.5%
0%
10.3%
6.6%
The percentage of patients in PsA-II achieving ACR20 response through Week 24 is shown below in Figure 2.
Figure 2: Percent of Patients Achieving ACR20 Responsea in PsA-II Study Through Week 24 (Day 169)
Results were generally consistent across the ACR components in Study PsA-I and PsA-II.
Improvements in enthesitis and dactylitis were seen with ORENCIA treatment at Week 24 in both PsA-I and PsA-II.
Physical Function Response
In study PsA-I, there was a higher proportion of patients with at least a 0.30 decrease from baseline in Health Assessment Questionnaire-Disability Index (HAQ-DI) score at Week 24, with an estimated difference for ORENCIA 10 mg/kg (weight range-based dosing as described above) (45.0%) vs. placebo (19.0%) of 26.1 (95% confidence interval: 6.8, 45.5). In study PsA-II, the proportion of patients with at least a 0.35 decrease from baseline in HAQ-DI on ORENCIA was 31%, as compared to 24% on placebo (estimated difference: 7%; 95% confidence interval: -1%, 16%). There was a higher adjusted mean change from baseline in HAQ-DI on ORENCIA (-0.33) vs. placebo (-0.20) at Week 24, with an estimated difference of -0.13 (95% confidence interval: -0.25, -0.01).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
For Intravenous Infusion
ORENCIA® (abatacept) for Injection is a lyophilized powder for intravenous infusion after reconstitution and dilution. It is supplied as an individually packaged, single-use vial with a silicone-free disposable syringe, providing 250 mg of abatacept in a 15-mL vial:
NDC: 0003-2187-10: in a clamshell presentation
NDC: 0003-2187-13: in a carton presentation
For Subcutaneous Injection
ORENCIA® (abatacept) Injection and ORENCIA® ClickJect (abatacept) are solutions for subcutaneous administration.
Prefilled Syringe
ORENCIA Injection, 50 mg/0.4 mL, 87.5 mg/0.7 mL, and 125 mg/mL, is supplied as single-dose disposable prefilled glass syringes with BD UltraSafe Passive™ needle guard and flange extenders.
The Type I glass syringe has a coated stopper and fixed stainless steel needle (5 bevel, 29-gauge thin wall, ½-inch needle) covered with a rigid needle shield. The prefilled syringe provides abatacept in the following packages:
NDC: 0003-2814-11 (50 mg/0.4 mL): pack of 4 syringes with a passive needle safety guard
NDC: 0003-2818-11 (87.5 mg/0.7 mL): pack of 4 syringes with a passive needle safety guard
NDC: 0003-2188-11 (125 mg/mL): pack of 4 syringes with a passive needle safety guard
ClickJect Autoinjector
ORENCIA ClickJect, 125 mg/mL, is supplied as a single-dose disposable prefilled autoinjector. The Type I glass syringe contained in the autoinjector has a coated stopper and fixed stainless steel needle (5 bevel, 27-gauge special thin wall, ½-inch needle) covered with a rigid needle shield. The autoinjector provides 125 mg of abatacept in 1 mL and is provided in the following package:
NDC: 0003-2188-51: pack of 4 autoinjectors
Storage
ORENCIA lyophilized powder supplied in a vial should be refrigerated at 2°C to 8°C (36°F to 46°F). Do not use beyond the expiration date on the vial. Protect the vials from light by storing in the original package until time of use.
ORENCIA solution supplied in a prefilled syringe or ClickJect autoinjector should be refrigerated at 2°C to 8°C (36°F to 46°F). Do not use beyond the expiration date on the prefilled syringe or autoinjector. Protect from light by storing in the original package until time of use. Do not allow the prefilled syringe or autoinjector to freeze.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Concomitant Use With Biologic Medications for RA
Inform patients that they should not receive ORENCIA treatment concomitantly with a TNF antagonist, such as adalimumab, etanercept, and infliximab because such combination therapy may increase their risk for infections [see Indications and Usage (1.4), Warnings and Precautions (5.1), and Drug Interactions (7.1)], and that they should not receive ORENCIA concomitantly with other biologic RA therapy, such as anakinra because there is not enough information to assess the safety and efficacy of such combination therapy [see Indications and Usage (1.4), Drug Interactions (7.2)].
Hypersensitivity
Instruct patients to immediately tell their healthcare professional if they experience symptoms of an allergic reaction during or for the first day after the administration of ORENCIA [see Warnings and Precautions (5.2)].
Infections
Ask patients if they have a history of recurrent infections, have underlying conditions which may predispose them to infections, or have chronic, latent, or localized infections. Ask patients if they have had tuberculosis (TB), a positive skin test for TB, or recently have been in close contact with someone who has had TB. Instruct patients that they may be tested for TB before they receive ORENCIA. Inform patients to tell their healthcare professional if they develop an infection during therapy with ORENCIA [see Warnings and Precautions (5.3)].
Immunizations
Inform patients that live vaccines should not be given concurrently with ORENCIA or within 3 months of its discontinuation. Inform caregivers of patients with juvenile idiopathic arthritis that the patient should be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating ORENCIA therapy and to discuss with their healthcare provider how best to handle future immunizations once ORENCIA therapy has been initiated [see Warnings and Precautions (5.4)].
Pregnancy and Nursing Mothers
Inform patients that ORENCIA has not been studied in pregnant women or nursing mothers so the effects of ORENCIA on pregnant women or nursing infants are not known. Instruct patients to tell their healthcare professional if they are pregnant, become pregnant, or are thinking about becoming pregnant [see Use in Specific Populations (8.1)]. Instruct patients to tell their healthcare professional if they plan to breastfeed their infant [see Use in Specific Populations (8.2)].
Blood Glucose Testing
Intravenous Administration
Ask patients if they have diabetes. Maltose is contained in ORENCIA for intravenous administration and can give falsely elevated blood glucose readings with certain blood glucose monitors on the day of ORENCIA infusion. If a patient is using such a monitor, advise the patient to discuss with their healthcare professional methods that do not react with maltose [see Drug Interactions (7.3)].
Disposal of Prefilled Syringes and ClickJect Autoinjectors
Advise patients to follow disposal instructions in the Instructions for Use. A puncture-resistant container for disposal of needles and syringes should be used. Instruct patients that they will need to follow their community guidelines for the correct way to dispose of their sharps disposal container. Instruct patients not to recycle their used sharps disposal container.
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION
ORENCIA® (oh-REN-see-ah)
(abatacept)
for injection, for intravenous use
ORENCIA® (oh-REN-see-ah)
(abatacept)
injection, for subcutaneous useWhat is ORENCIA?
ORENCIA is a prescription medicine that reduces signs and symptoms in:- adults with moderate to severe rheumatoid arthritis (RA), including those who have not been helped enough by other medicines for RA. ORENCIA may prevent further damage to your bones and joints and may help your ability to perform daily activities. In adults, ORENCIA may be used alone or with other RA treatments other than tumor necrosis factor (TNF) antagonists.
- patients 2 years of age and older with moderate to severe polyarticular juvenile idiopathic arthritis (JIA). ORENCIA may be used alone or with methotrexate.
- adults with active psoriatic arthritis (PsA). In adults, ORENCIA can be used alone or with other PsA treatments.
It is not known if ORENCIA is safe and effective in children under 2 years of age.
It is not known if ORENCIA is safe and effective in children for uses other than juvenile idiopathic arthritis.
Before you use ORENCIA, tell your healthcare provider about all of your medical conditions, including if you:
- have any kind of infection even if it is small (such as an open cut or sore), or an infection that is in your whole body (such as the flu). If you have an infection when taking ORENCIA, you may have a higher chance for getting serious side effects.
- have an infection that will not go away or an infection that keeps coming back.
- are allergic to abatacept or any of the ingredients in ORENCIA. See the end of this Patient Information leaflet for a complete list of ingredients in ORENCIA.
- have or have had inflammation of your liver due to an infection (viral hepatitis). Before you use ORENCIA, your healthcare provider may examine you for hepatitis.
- have had a lung infection called tuberculosis (TB), a positive skin test for TB, or you recently have been in close contact with someone who has had TB. Before you use ORENCIA, your healthcare provider may examine you for TB or perform a skin test. Symptoms of TB may include:
- o a cough that does not go away
- o weight loss
- o fever
- o night sweats
- are scheduled to have surgery.
- recently received a vaccination or are scheduled for a vaccination. If you are receiving ORENCIA, and for 3 months after you stop receiving ORENCIA, you should not receive live vaccines.
- have a history of a breathing problem called chronic obstructive pulmonary disease (COPD).
- have diabetes and use a blood glucose monitor to check your blood sugar (blood glucose) levels. ORENCIA for intravenous infusion (given through a needle placed in a vein) contains maltose, a type of sugar, that can give false high blood sugar readings with certain types of blood glucose monitors on the day of ORENCIA infusion. Your healthcare provider may tell you to use a different way to monitor your blood sugar levels.
- ORENCIA for subcutaneous injection (injected under the skin) does not contain maltose. You do not need to change your blood sugar monitoring if you are taking ORENCIA subcutaneously.
-
are pregnant or plan to become pregnant. It is not known if ORENCIA can harm your unborn baby. If you took ORENCIA during pregnancy, talk to your healthcare provider before your baby receives any vaccines.
- o
Bristol-Myers Squibb Company has a registry for pregnant women exposed to ORENCIA. The purpose of this registry is to check the health of the pregnant mother and her child. Women are encouraged to call the registry themselves or ask their healthcare provider to contact the registry for them by calling 1-877-311-8972.
- are breastfeeding or plan to breastfeed. It is not known if ORENCIA passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ORENCIA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
ORENCIA may affect the way other medicines work, and other medicines may affect the way ORENCIA works causing serious side effects.
Especially tell your healthcare provider if you take other biologic medicines to treat RA, JIA or PsA that may affect your immune system, such as:
- Enbrel® (etanercept)
- Humira® (adalimumab)
- Remicade® (infliximab)
- Kineret® (anakinra)
- Rituxan® (rituximab)
- Simponi® (golimumab)
- Cimzia® (certolizumab pegol)
- Actemra® (tocilizumab)
You may have a higher chance of getting a serious infection if you take ORENCIA with other biologic medicines for your RA, JIA, or PsA.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new prescription.
How should I use ORENCIA?
- You may receive ORENCIA given by a healthcare provider through a vein in your arm (IV or intravenous infusion). It takes about 30 minutes to give you the full dose of medicine. You will then receive ORENCIA 2 weeks and 4 weeks after the first dose and then every 4 weeks.
- You may also receive ORENCIA as an injection under your skin (subcutaneous). For home use, ORENCIA comes in a prefilled syringe or prefilled ClickJect autoinjector. Your healthcare provider will prescribe the type that is best for you. If your healthcare provider decides that you or a caregiver can give your injections of ORENCIA prefilled syringes or ORENCIA ClickJect autoinjectors at home, you or your caregiver should receive training on the right way to prepare and inject ORENCIA. Do not try to inject ORENCIA until you have been shown the right way to give the injections by your healthcare provider.
- Your healthcare provider will tell you how much ORENCIA to use and when to use it.
-
See the Instructions for Use at the end of this Patient Information leaflet for instructions about the right way to prepare and give your ORENCIA injections at home.
What are the possible side effects of ORENCIA?
ORENCIA can cause serious side effects including:
- infections. ORENCIA can make you more likely to get infections or make the infection that you have get worse. Some people have died from these infections. Call your healthcare provider right away if you have any symptoms of an infection. Symptoms of an infection may include:
- o fever
- o feel very tired
- o have a cough
- o have flu-like symptoms
- o warm, red, or painful skin
- allergic reactions. Allergic reactions can happen to people who use ORENCIA. Call your healthcare provider or go to the emergency room right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- o hives
- o swollen face, eyelids, lips, or tongue
- o trouble breathing
- hepatitis B infection in people who carry the virus in their blood. If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use ORENCIA. Your healthcare provider may do a blood test before you start treatment with ORENCIA.
- vaccinations. You should not receive ORENCIA with certain types of vaccines (live vaccines). ORENCIA may also cause some vaccinations to be less effective. Talk with your healthcare provider about your vaccination plans.
- breathing problems in people with Chronic Obstructive Pulmonary Disease (COPD). Some people may get certain respiratory problems more often if they receive ORENCIA and have COPD. Symptoms of respiratory problems include:
- o COPD that becomes worse
- o cough
- o trouble breathing
- cancer (malignancies). Certain kinds of cancer have been reported in people using ORENCIA. It is not known if ORENCIA increases your chance of getting certain kinds of cancer.
Common side effects of ORENCIA include:
- headache
- upper respiratory tract infection
- sore throat
- nausea
In children and adolescents, other side effects may include:
- diarrhea
- cough
- fever
- abdominal pain
These are not all the possible side effects of ORENCIA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ORENCIA?
- Store ORENCIA in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Keep ORENCIA in the original package and out of the light.
- Do not freeze ORENCIA.
- Safely throw away medicine that is out of date or no longer needed.
Keep ORENCIA and all medicines out of the reach of children.
General information about the safe and effective use of ORENCIA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ORENCIA for a condition for which it was not prescribed. Do not give ORENCIA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about ORENCIA that is written for health professionals.
What are the ingredients in ORENCIA?
Active ingredient: abatacept
Intravenous inactive ingredients: maltose, monobasic sodium phosphate, sodium chloride for administration
Subcutaneous inactive ingredients: sucrose, poloxamer 188, monobasic sodium phosphate monohydrate, dibasic sodium phosphate anhydrous, water for injection
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA, U.S. License Number 1713All other trademarks are property of their respective owners.
For more information, go to www.ORENCIA.com or call 1-800-ORENCIA.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Rev June/2017
-
INSTRUCTIONS FOR USE
ORENCIA® (oh-REN-see-ah)
(abatacept)
Prefilled Syringe with BD UltraSafe Passive™ Needle GuardStep 1: Preparing for an ORENCIA Injection
Step 2: Examine the Prefilled Syringe
Step 3: Check the Dose on the Prefilled Syringe
Step 4: Choose and Prepare an Injection Site
Step 5: Inject Your Dose of ORENCIA
Step 6: After the Injection
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA, U.S. License Number 1713
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
ORENCIA is a registered trademark of Bristol-Myers Squibb Company.
BD UltraSafe Passive™ is a trademark of Becton, Dickinson and Company.
Revised March 2017 -
INSTRUCTIONS FOR USE
ORENCIA®ClickJect™ (oh-REN-see-ah)
(abatacept)
Prefilled AutoinjectorStep 1: Prepare Your Autoinjector
Step 2: Prepare for Injection
Step 3: Inject Your Dose
Step 4: After the Injection
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA, U.S. License Number 1713This Instructions for Use has been approved by the U.S. Food and Drug Administration.
ORENCIA is a registered trademark and ClickJect is a trademark of Bristol-Myers Squibb Company.
Revised March 2017
-
ORENCIA 250 mg/vial for Injection Representative Packaging
See How Supplied section for a complete list of available packages of ORENCIA.
Rx only
NDC: 0003-2187-13
ORENCIA®
(abatacept)
Lyophilized Powder for IV Infusion
250 mg/vial
ONLY USE THE SILICONE-FREE DISPOSABLE SYRINGE INCLUDED IN THE PACKAGE FOR RECONSTITUTION
Bristol-Myers Squibb Company
Princeton, New Jersey 08543 USA
US License No. 1713 -
ORENCIA 50 mg/0.4 mL Injection Representative Packaging
Bristol-Myers Squibb
NDC: 0003-2814-11
4 Single-Dose Prefilled Syringes with BD UltraSafe Passive™ Needle Guard
ORENCIA®
(abatacept)
Injection
50 mg/0.4mL
Single-Dose Prefilled Syringe with BD UltraSafe Passive™ Needle Guard
FOR SUBCUTANEOUS USE ONLY
WARNING: Keep out of reach of children
Discard each syringe after use
Rx only -
ORENCIA 87.5 mg/0.7 mL Injection Representative Packaging
Bristol-Myers Squibb
NDC: 0003-2818-11
4 Single-Dose Prefilled Syringes with BD UltraSafe Passive™ Needle Guard
ORENCIA®
(abatacept)
Injection
87.5 mg/0.7mL
Single-Dose Prefilled Syringe with BD UltraSafe Passive™ Needle Guard
FOR SUBCUTANEOUS USE ONLY
WARNING: Keep out of reach of children
Discard each syringe after use
Rx only -
ORENCIA 125 mg/mL Injection Representative Packaging
Bristol-Myers Squibb
NDC: 0003-2188-11
4 Single-Dose Prefilled Syringes with BD UltraSafe Passive™ Needle Guard
ORENCIA®
(abatacept)
Injection
125 mg/mL
Single-Dose Prefilled Syringe with BD UltraSafe Passive™ Needle Guard
FOR SUBCUTANEOUS USE ONLY
WARNING: Keep out of reach of children
Discard each syringe after use
Rx only -
ORENCIA 125 mg/mL ClickJect Representative Packaging
Bristol-Myers Squibb
NDC: 0003-2188-51
4 Single-Dose Prefilled Autoinjectors
ORENCIA® ClickJect™
(abatacept)
Injection
125 mg/mL
Single-Dose Autoinjector
FOR SUBCUTANEOUS USE ONLY
Refrigerate immediately
WARNING: Keep out of reach of children
Discard each autoinjector after use
Open from this side Rx only -
INGREDIENTS AND APPEARANCE
ORENCIA
abatacept injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-2187 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength abatacept (UNII: 7D0YB67S97) (abatacept - UNII:7D0YB67S97) abatacept 250 mg in 15 mL Inactive Ingredients Ingredient Name Strength maltose monohydrate (UNII: DM477EE40D) 500 mg in 15 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) 17.2 mg in 15 mL sodium chloride (UNII: 451W47IQ8X) 14.6 mg in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-2187-10 1 in 1 CELLO PACK 01/01/2009 1 15 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package 2 NDC: 0003-2187-13 1 in 1 CARTON 01/01/2009 2 15 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125118 01/01/2009 ORENCIA
abatacept injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-2188 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength abatacept (UNII: 7D0YB67S97) (abatacept - UNII:7D0YB67S97) abatacept 125 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 0.838 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) 0.286 mg in 1 mL poloxamer 188 (UNII: LQA7B6G8JG) 8 mg in 1 mL sucrose (UNII: C151H8M554) 170 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-2188-11 4 in 1 CARTON 10/01/2013 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0003-2188-21 1 in 1 CARTON 10/01/2013 2 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC: 0003-2188-91 1 in 1 CARTON 10/01/2013 3 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 4 NDC: 0003-2188-51 4 in 1 CARTON 06/07/2016 4 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 5 NDC: 0003-2188-50 1 in 1 CARTON 07/05/2016 5 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 6 NDC: 0003-2188-90 1 in 1 CARTON 07/12/2016 6 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125118 07/29/2011 ORENCIA
abatacept injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-2814 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ABATACEPT (UNII: 7D0YB67S97) (ABATACEPT - UNII:7D0YB67S97) ABATACEPT 50 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 0.335 mg in 0.4 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.114 mg in 0.4 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 3.2 mg in 0.4 mL SUCROSE (UNII: C151H8M554) 68 mg in 0.4 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-2814-11 4 in 1 CARTON 03/30/2017 1 0.4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125118 07/29/2011 ORENCIA
abatacept injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0003-2818 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ABATACEPT (UNII: 7D0YB67S97) (ABATACEPT - UNII:7D0YB67S97) ABATACEPT 87.5 mg in 0.7 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 0.587 mg in 0.7 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.200 mg in 0.7 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 5.6 mg in 0.7 mL SUCROSE (UNII: C151H8M554) 119 mg in 0.7 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0003-2818-11 4 in 1 CARTON 03/30/2017 1 0.7 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125118 07/29/2011 Labeler - E.R. Squibb & Sons, L.L.C. (011550092)
Trademark Results [ORENCIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ORENCIA 78756090 3184992 Live/Registered |
Bristol-Myers Squibb Company 2005-11-17 |
 ORENCIA 78465053 not registered Dead/Abandoned |
Bristol-Myers Squibb Company 2004-08-10 |
 ORENCIA 76213107 2948497 Live/Registered |
BRISTOL-MYERS SQUIBB COMPANY 2001-02-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.