GLYBURIDE TABLETS USP Rx only
Glyburide by
Drug Labeling and Warnings
Glyburide by is a Prescription medication manufactured, distributed, or labeled by Preferred Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GLYBURIDE- glyburide tablet
Preferred Pharmaceuticals Inc.
----------
GLYBURIDE TABLETS USP
Rx only
DESCRIPTION
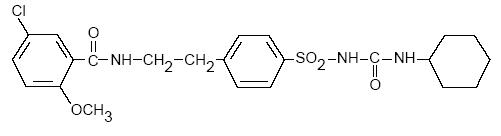
- Glyburide tablets USP contain glyburide, which is an oral blood-glucose-lowering drug of the sulfonylurea class. Glyburide is a white, crystalline compound, formulated as Glyburide tablets of 1.25, 2.5, and 5 mg strengths for oral administration. Inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate, and magnesium stearate. In addition, the 1.25 mg contains FD&C blue #1 aluminum lake and the 2.5 mg contains D&C yellow #10 aluminum lake. The chemical name for glyburide is 1-[ [p-[2-(5-chloro-o-anisamido)ethyl]phenyl]-sulfonyl]-3-cyclohexylurea and the molecular weight is 493.99. The structural formula is represented below.
- C 23 H 28 ClN 3 O 5 S M.W. 493.99
CLINICAL PHARMACOLOGY
Actions
- Glyburide appears to lower the blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. The mechanism by which glyburide lowers blood glucose during long-term administration has not been clearly established. With chronic administration in Type II diabetic patients, the blood glucose lowering effect persists despite a gradual decline in the insulin secretory response to the drug. Extrapancreatic effects may be involved in the mechanism of action of oral sulfonylurea hypoglycemic drugs. The combination of glyburide and metformin may have a synergistic effect, since both agents act to improve glucose tolerance by different but complementary mechanisms.
Pharmacokinetics
- Single dose studies with glyburide tablets in normal subjects demonstrate significant absorption of glyburide within one hour, peak drug levels at about four hours, and low but detectable levels at twenty-four hours. Mean serum levels of glyburide, as reflected by areas under the serum concentration-time curve, increase in proportion to corresponding increases in dose. Multiple dose studies with glyburide in diabetic patients demonstrate drug level concentration-time curves similar to single dose studies, indicating no buildup of drug in tissue depots. The decrease of glyburide in the serum of normal healthy individuals is biphasic; the terminal half-life is about 10 hours. In single dose studies in fasting normal subjects, the degree and duration of blood glucose lowering is proportional to the dose administered and to the area under the drug level concentration-time curve. The blood glucose lowering effect persists for 24 hours following single morning doses in nonfasting diabetic patients. Under conditions of repeated administration in diabetic patients, however, there is no reliable correlation between blood drug levels and fasting blood glucose levels. A one year study of diabetic patients treated with glyburide showed no reliable correlation between administered dose and serum drug level.
INDICATIONS AND USAGE
- Glyburide tablets USP are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups.
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glyburide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
PRECAUTIONS
General
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with glyburide or any other anti-diabetic drug.
Hypoglycemia
- All sulfonylureas including glyburide are capable of producing severe hypoglycemia. Proper patient selection and dosage and instructions are important to avoid hypoglycemic episodes. Renal or hepatic insufficiency may cause elevated drug levels of glyburide and the latter may also diminish gluconeogenic capacity, both of which increase the risk of serious hypoglycemic reactions. Elderly, debilitated or malnourished patients, and those with adrenal or pituitary insufficiency, are particularly susceptible to the hypoglycemic action of glucose-lowering drugs. Hypoglycemia may be difficult to recognize in the elderly and in people who are taking beta-adrenergic blocking drugs. Hypoglycemia is more likely to occur when caloric intake is deficient, after severe or prolonged exercise, when alcohol is ingested, or when more than one glucose lowering drug is used. The risk of hypoglycemia may be increased with combination therapy.
Loss of Control of Blood Glucose
- When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection or surgery, a loss of control may occur. At such times it may be necessary to discontinue glyburide and administer insulin.
Hemolytic Anemia
- Treatment of patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents can lead to hemolytic anemia. Because glyburide belongs to the class of sulfonylurea agents, caution should be used in patients with G6PD deficiency and a non-sulfonylurea alternative should be considered. In postmarketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
Information for Patients
- Patients should be informed of the potential risks and advantages of glyburide and of alternative modes of therapy. They also should be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of urine and/or blood glucose.
Physician Counseling Information for Patients
- In initiating treatment for type 2 diabetes, diet should be emphasized as the primary form of treatment. Caloric restriction and weight loss are essential in the obese diabetic patient. Proper dietary management alone may be effective in controlling the blood glucose and symptoms of hyperglycemia. The importance of regular physical activity should also be stressed, and cardiovascular risk factors should be identified and corrective measures taken where possible. Use of glyburide or other antidiabetic medications must be viewed by both the physician and patient as a treatment in addition to diet and not as a substitution or as a convenient mechanism for avoiding dietary restraint. Furthermore, loss of blood glucose control on diet alone may be transient, thus requiring only short-term administration of glyburide or other antidiabetic medications. Maintenance or discontinuation of glyburide or other antidiabetic medications should be based on clinical judgment using regular clinical and laboratory evaluations.
Laboratory Tests
- Therapeutic response to glyburide tablets should be monitored by frequent urine glucose tests and periodic blood glucose tests. Measurement of glycosylated hemoglobin levels may be helpful in some patients.
Drug Interactions
- The hypoglycemic action of sulfonylureas may be potentiated by certain drugs including non-steroidal anti-inflammatory agents and other drugs that are highly protein bound, salicylates, sulfonamides, chloramphenicol, probenecid, coumarins, monoamine oxidase inhibitors, and beta adrenergic blocking agents. When such drugs are administered to a patient receiving glyburide, the patient should be observed closely for hypoglycemia. When such drugs are withdrawn from a patient receiving glyburide, the patient should be observed closely for loss of control.
Metformin
- In a single-dose interaction study in NIDDM subjects, decreases in glyburide AUC and C max were observed, but were highly variable. The single-dose nature of this study and the lack of correlation between glyburide blood levels and pharmacodynamic effects, makes the clinical significance of this interaction uncertain. Coadministration of glyburide and metformin did not result in any changes in either metformin pharmacokinetics or pharmacodynamics.
Colesevelam
- Concomitant administration of colesevelam and glyburide resulted in reductions in glyburide AUC and C max of 32% and 47%, respectively. The reductions in glyburide AUC and C max were 20% and 15%, respectively when administered 1 hour before, and not significantly changed (-7% and 4%, respectively) when administered 4 hours before colesevelam.
Topiramate
- A drug-drug interaction study conducted in patients with type 2 diabetes evaluated the steady-state pharmacokinetics of glyburide (5 mg/day) alone and concomitantly with topiramate (150 mg/day). There was a 22% decrease in C max and a 25% reduction in AUC 24 for glyburide during topiramate administration. Systemic exposure (AUC) of the active metabolites, 4-trans-hydroxy-glyburide (M1) and 3-cis-hydroxyglyburide (M2), was also reduced by 13% and 15%, and C max was reduced by 18% and 25%, respectively. The steady-state pharmacokinetics of topiramate were unaffected by concomitant administration of glyburide.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Studies in rats at doses up to 300 mg/kg/day for 18 months showed no carcinogenic effects. Glyburide is nonmutagenic when studied in the Salmonella microsome test (Ames test) and in the DNA damage/alkaline elution assay. No drug related effects were noted in any of the criteria evaluated in the two year oncogenicity study of glyburide in mice.
Pregnancy
Teratogenic Effects
Pregnancy Category B
- Reproduction studies have been performed in rats and rabbits at doses up to 500 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to glyburide. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
- Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives. If glyburide is used during pregnancy, it should be discontinued at least two weeks before the expected delivery date.
Nursing Mothers
- Although it is not known whether glyburide is excreted in human milk, some sulfonylurea drugs are known to be excreted in human milk. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. If the drug is discontinued, and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Geriatric Use
- Elderly patients are particularly susceptible to the hypoglycemic action of glucose lowering drugs. Hypoglycemia may be difficult to recognize in the elderly (see PRECAUTIONS ). The initial and maintenance dosing should be conservative to avoid hypoglycemic reactions (see DOSAGE AND ADMINISTRATION ).
ADVERSE REACTIONS
Hypoglycemia: See PRECAUTIONS and OVERDOSAGE Sections.
Gastrointestinal Reactions: Cholestatic jaundice and hepatitis may occur rarely which may progress to liver failure; glyburide tablets should be discontinued if this occurs.
- Liver function abnormalities, including isolated transaminase elevations, have been reported.
Dermatologic Reactions: Allergic skin reactions, e.g. , pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions occurred in 1.5% of treated patients during clinical trials. These may be transient and may disappear despite continued use of glyburide; if skin reactions persist, the drug should be discontinued.
- Porphyria cutanea tarda and photosensitivity reactions have been reported with sulfonylureas.
Hematologic Reactions: Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia (see PRECAUTIONS ), aplastic anemia, and pancytopenia have been reported with sulfonylureas.
Metabolic Reactions: Hepatic porphyria and disulfiram-like reactions have been reported with sulfonylureas; however, hepatic porphyria has not been reported with glyburide and disulfiram-like reactions have been reported very rarely.
- Cases of hyponatremia have been reported with glyburide and all other sulfonylureas, most often in patients who are on other medications or have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone. The syndrome of inappropriate antidiuretic hormone (SIADH) secretion has been reported with certain other sulfonylureas, and it has been suggested that these sulfonylureas may augment the peripheral (antidiuretic) action of ADH and/or increase release of ADH.
Other Reactions: Changes in accommodation and/or blurred vision have been reported with glyburide and other sulfonylureas. These are thought to be related to fluctuation in glucose levels.
- In addition to dermatologic reactions, allergic reactions such as angioedema, arthralgia, myalgia and vasculitis have been reported.
OVERDOSAGE
- Overdosage of sulfonylureas, including glyburide tablets, can produce hypoglycemia. Mild hypoglycemic symptoms, without loss of consciousness or neurological findings, should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate which will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery.
How Supplied
Glyburide tablets USP, 5 mg are white to off-white circular biconvex tablets, debossed with the score line, logo "OP" andproduct ID No. "56"on one side and "5" on the other side. They are supplied as follows:
- NDC 68788-7970-3 bottles of 30
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Keep container tightly closed.
DOSAGE AND ADMINISTRATION
There is no fixed dosage regimen for the management of diabetes mellitus with glyburide tablets. In addition to the usual monitoring of urinary glucose, the patient's blood glucose must also be monitored periodically to determine the minimum effective dose for the patient; to detect primary failure, i.e. , inadequate lowering of blood glucose at the maximum recommended dose of medication; and to detect secondary failure, i.e. , loss of adequate blood glucose lowering response after an initial period of effectiveness. Glycosylated hemoglobin levels may also be of value in monitoring the patient's response to therapy.
Short-term administration of glyburide tablets may be sufficient during periods of transient loss of control in patients usually controlled well on diet.
Usual Starting Dose
- The usual starting dose of glyburide tablets is 2.5 to 5 mg daily, administered with breakfast or the first main meal. Those patients who may be more sensitive to hypoglycemic drugs should be started at 1.25 mg daily. (See PRECAUTIONS section for patients at increased risk.) Failure to follow an appropriate dosage regimen may precipitate hypoglycemia. Patients who do not adhere to their prescribed dietary and drug regimen are more prone to exhibit unsatisfactory response to therapy.
Transfer From Other Hypoglycemic Therapy Patients Receiving Other Oral Antidiabetic Therapy
- Transfer of patients from other oral antidiabetic regimens to glyburide tablets should be done conservatively and the initial daily dose should be 2.5 to 5 mg. When transferring patients from oral hypoglycemic agents other than chlorpropamide to glyburide tablets, no transition period and no initial or priming dose are necessary. When transferring patients from chlorpropamide, particular care should be exercised during the first two weeks because the prolonged retention of chlorpropamide in the body and subsequent overlapping drug effects may provoke hypoglycemia.
Patients Receiving Insulin
- Some Type II diabetic patients being treated with insulin may respond satisfactorily to glyburide tablets. If the insulin dose is less than 20 units daily, substitution of glyburide tablets 2.5 to 5 mg as a single daily dose may be tried. If the insulin dose is between 20 and 40 units daily, the patient may be placed directly on glyburide tablets 5 mg daily as a single dose. If the insulin dose is more than 40 units daily, a transition period is required for conversion to glyburide tablets. In these patients, insulin dosage is decreased by 50% and glyburide tablets 5 mg daily is started. Please refer to Titration to Maintenance Dose for further explanation.
Titration to Maintenance Dose
- The usual maintenance dose is in the range of 1.25 to 20 mg daily, which may be given as a single dose or in divided doses (see Dosage Interval section). Dosage increases should be made in increments of no more than 2.5 mg at weekly intervals based upon the patient's blood glucose response.
Concomitant Glyburide and Metformin Therapy
- Glyburide tablets should be added gradually to the dosing regimen of patients who have not responded to the maximum dose of metformin monotherapy after four weeks (see Usual Starting Dose and Titration to Maintenance Dose ). Refer to metformin package insert.
Dosage Interval
- Once-a-day therapy is usually satisfactory. Some patients, particularly those receiving more than 10 mg daily, may have a more satisfactory response with twice-a-day dosage.
Specific Patient Populations
Glyburide is not recommended for use in pregnancy or for use in pediatric patients.
In elderly patients, debilitated or malnourished patients, and patients with impaired renal or hepatic function, the initial and maintenance dosing should be conservative to avoid hypoglycemic reactions (see PRECAUTIONS section).
Glyburide tablets USP, 5 mg are white to off-white circular biconvex tablets, debossed with the score line, logo "OP" andproduct ID No. "56"on one side and "5" on the other side. They are supplied as follows:
NDC 0121-0931-90 bottles of 100
NDC 0121-0931-92 bottles of 1000
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Keep container tightly closed.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured by:
Orient Pharma Co., Ltd.
Yunlin, Taiwan
Distributed by:
Pharmaceutical Associates, Inc.
1700 Perimeter Road,
Greenville, SC 29605
Revised 06/20
Repackaged By: Preferred Pharmaceuticals Inc.
| GLYBURIDE
glyburide tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | REPACK(68788-7970) | |