Sovereign Silver Skin Care by Natural Immunogenics Corp. Homeopathic Drug
Sovereign Silver Skin Care by
Drug Labeling and Warnings
Sovereign Silver Skin Care by is a Homeopathic medication manufactured, distributed, or labeled by Natural Immunogenics Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
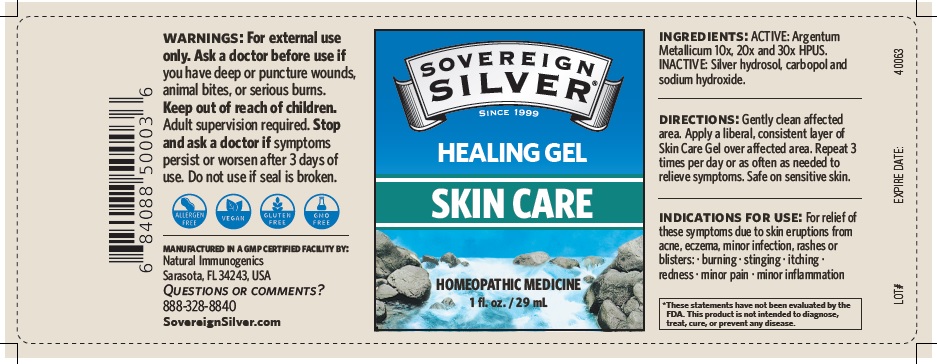
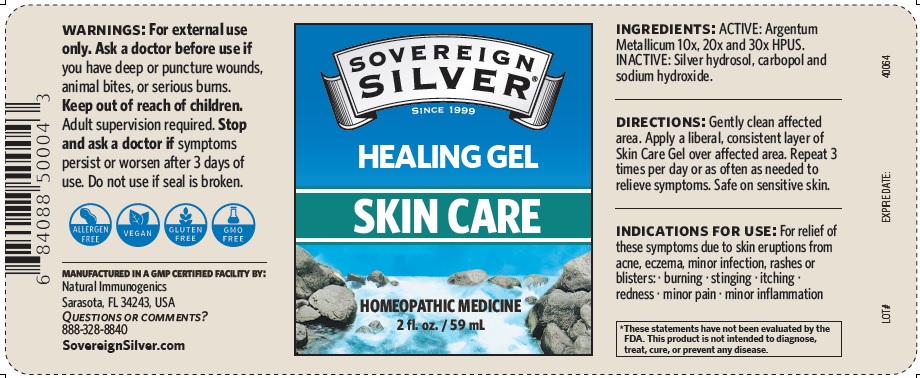
SOVEREIGN SILVER SKIN CARE- argentum metallicum gel
Natural Immunogenics Corp.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Homeopathic Drug
Warnings
For external use only.
Ask a doctor before use if you have deep or puncture wounds, animal bites or serious burns.
Stop and ask a doctor if symptoms persist or worsen after 3 days of use. Do not use if
seal is broken
Directions
Gently clean affected area.
Apply a liberal, consistent layer of Skin Care Gel over a affected area. Repeat 3 times per day or as often as needed to relieve symptoms. Safe on sensitive skin.
| SOVEREIGN SILVER SKIN CARE
argentum metallicum gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Natural Immunogenics Corp. (048744085) |
| Registrant - Natural Immunogenics Corp. (048744085) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Natural Immunogenics Corp. | 048744085 | manufacture(52166-014) | |