FUROSEMIDE injection, solution
Furosemide by
Drug Labeling and Warnings
Furosemide by is a Prescription medication manufactured, distributed, or labeled by Phlow Corporation, Fresenius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FUROSEMIDE INJECTION safely and effectively. See full prescribing information for FUROSEMIDE INJECTION.
FUROSEMIDE INJECTION (furosemide), for intravenous or intramuscular use
Initial U.S. Approval: 1982INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Edema:
- Initial dose is 20 mg to 40 mg once given intramuscularly or intravenously. The intravenous dose should be administered slowly over 1 minute to 2 minutes (2.2)
- If needed, a second dose may be administered 2 hours after the first dose (2.2)
Acute Pulmonary Edema:
- Initial dose is 40 mg injected slowly intravenously over 1 minute to 2 minutes (2.2)
- If needed, a second dose is 80 mg injected intravenously slowly in 1 minute to 2 minutes (2.2)
Pediatric Dosage:
- The initial dose in pediatric patients is 1 mg/kg body weight once given slowly intramuscularly or intravenously. If needed, dosage may be increased by 1 mg/kg not sooner than 2 hours after the previous dose, until the desired diuretic effect has been obtained. Doses greater than 6 mg/kg body weight are not recommended (2.3)
DOSAGE FORMS AND STRENGTHS
Injection: Furosemide Injection, USP is supplied as a sterile, colorless solution as
- 20 mg/2 mL (10 mg/mL) in a single-dose vial (3)
WARNINGS AND PRECAUTIONS
- Fluid, Electrolyte, and Metabolic Abnormalities: Monitor serum electrolytes, CO2, BUN, creatinine, glucose, and uric acid (5.1)
- Worsening Renal Function: Monitor for dehydration and azotemia. (5.2)
- Ototoxicity: Avoid rapid injection and higher than recommended doses. (5.3, 7.1)
- Acute Urinary Retention: Monitor patients with symptoms of urinary retention. (5.4)
ADVERSE REACTIONS
Most common adverse reactions are related to fluid and electrolyte imbalance (6)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Aminoglycoside antibiotics: Increased potential ototoxicity of the antibiotics. Avoid combination (7.1)
- Ethacrynic acid: Risk of ototoxicity. Avoid combination (7.1)
- Salicylates: Risk of salicylate toxicity (7.1)
- Cisplatin and nephrotoxic drugs: Risk of ototoxicity and nephrotoxicity (7.1)
- Lithium: Risk of lithium toxicity (7.1)
- Renin-angiotensin inhibitors: Increased risk of hypotension and renal failure. (7.1)
- Adrenergic blocking drugs: Risk of potentiation (7.1)
- Drugs undergoing renal tubular secretion: Risk of toxicity potentiation (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Edema
1.2 Acute Pulmonary Edema
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
2.2 Recommended Dosage for Adults
2.3 Recommended Dosage for Pediatric Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fluid, Electrolyte, and Metabolic Abnormalities
5.2 Worsening Renal Function
5.3 Ototoxicity
5.4 Acute Urinary Retention
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effects of Furosemide on Other Drugs
7.2 Effects of Other Drugs on Furosemide
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
Inspect Furosemide Injection visually for particulate matter and discoloration before administration.
2.2 Recommended Dosage for Adults
Edema
Individualize therapy according to patient response.
The usual initial dose of furosemide is 20 mg to 40 mg given as a single-dose, injected intramuscularly or intravenously. Give the intravenous dose slowly (over 1 minute to 2 minutes). If needed, administer another dose in the same manner 2 hours later or increase the dose. The dose may be raised by 20 mg and administered not sooner than 2 hours after the previous dose until the desired diuretic effect has been obtained. Administer this individually determined single-dose once or twice daily.
If the physician elects to use high-dose parenteral therapy, add the furosemide to either 0.9% Sodium Chloride Injection USP, Lactated Ringer's Injection USP, or Dextrose Injection 5%, USP, after pH has been adjusted to above 5.5, and administer as a controlled intravenous infusion at a rate not greater than 4 mg/min. Furosemide Injection is a buffered alkaline solution with a pH of about 9 and the drug may precipitate at pH values below 7. Care must be taken to ensure that the pH of the prepared infusion solution is in the weakly alkaline to neutral range. Acid solutions, including other parenteral medications (e.g., labetalol, ciprofloxacin, amrinone, milrinone) must not be administered concurrently in the same infusion because they may cause precipitation of the furosemide.
2.3 Recommended Dosage for Pediatric Patients
The usual initial dose of Furosemide Injection (intravenously or intramuscularly) in pediatric patients is 1 mg/kg body weight administered slowly (over 1 minute to 2 minutes). If the diuretic response to the initial dose is not satisfactory, dosage may be increased by 1 mg/kg not sooner than 2 hours after the previous dose, until the desired diuretic effect has been obtained. Doses greater than 6 mg/kg body weight are not recommended.
The maximum dose for premature infants should not exceed 1 mg/kg/day [see Use in Specific Populations (8.4)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Fluid, Electrolyte, and Metabolic Abnormalities
Furosemide may cause fluid, electrolyte, and metabolic abnormalities such as hypovolemia, hypokalemia, azotemia, hyponatremia, hypochloremic alkalosis, hypomagnesemia, hypocalcemia, hyperglycemia, or hyperuricemia, particularly in patients receiving higher doses, patients with inadequate oral electrolyte intake, and in elderly patients. Excessive diuresis may cause dehydration and blood volume reduction with circulatory collapse and possibly vascular thrombosis and embolism, particularly in elderly patients. Serum electrolytes, CO2, BUN, creatinine, glucose, and uric acid should be monitored frequently during furosemide therapy.
In patients with hepatic cirrhosis and ascites, sudden alterations of fluid and electrolyte balance may precipitate hepatic encephalopathy and coma. Treatment in such patients is best initiated in the hospital with small doses and careful monitoring of the patient's clinical status and electrolyte balance.
5.2 Worsening Renal Function
Furosemide can cause dehydration and azotemia. If increasing azotemia and oliguria occur during treatment of severe progressive renal disease, furosemide should be discontinued [see Clinical Pharmacology (12.3)].
Furosemide use in the first year of life, especially in patients born pre-term, may precipitate nephrocalcinosis/nephrolithiasis. Therefore renal function must be monitored and renal ultrasonography performed in this age group [see Use in Specific Populations (8.4)].
5.3 Ototoxicity
Cases of tinnitus and reversible or irreversible hearing impairment and deafness have been reported. Reports usually indicate that furosemide ototoxicity is associated with rapid injection, severe renal impairment, the use of higher than recommended doses, hypoproteinemia or concomitant therapy with aminoglycoside antibiotics, ethacrynic acid, or other ototoxic drugs. If the physician elects to use high-dose parenteral therapy, controlled intravenous infusion is advisable (for adults, an infusion rate not exceeding 4 mg furosemide per minute has been used) [see Drug Interactions (7.1)].
Hearing loss in neonates, including premature neonates has been associated with the use of Furosemide Injection [see Use in Specific Populations (8.4)].
5.4 Acute Urinary Retention
In patients with severe symptoms of urinary retention (because of bladder emptying disorders, prostatic hyperplasia, urethral narrowing), the administration of furosemide can cause acute urinary retention related to increased production and retention of urine. Thus, these patients require careful monitoring, especially during the initial stages of treatment.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Fluid, Electrolyte, and Metabolic Abnormalities [see Warnings and Precautions (5.1)]
- Ototoxicity [see Warnings and Precautions (5.3)]
The following adverse reactions associated with the use of furosemide were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions are categorized below by organ system and listed by decreasing severity.
Gastrointestinal System Reactions: pancreatitis, jaundice (intrahepatic cholestatic jaundice), increased liver enzymes, anorexia, oral and gastric irritation, cramping, diarrhea, constipation, nausea, vomiting.
Systemic Hypersensitivity Reactions: severe anaphylactic or anaphylactoid reactions (e.g., with shock), systemic vasculitis, interstitial nephritis, necrotizing angiitis.
Central Nervous System Reactions: tinnitus and hearing loss, paresthesias, vertigo, dizziness, headache, blurred vision, xanthopsia.
Hematologic Reactions: aplastic anemia, thrombocytopenia, agranulocytosis, hemolytic anemia, leukopenia, anemia, eosinophilia.
Dermatologic-Hypersensitivity Reactions: toxic epidermal necrolysis, Stevens-Johnson Syndrome, erythema multiforme, drug rash with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis, exfoliative dermatitis, bullous pemphigoid, purpura, photosensitivity, rash.
Cardiovascular Reactions: orthostatic hypotension, increase in cholesterol and triglyceride serum levels.
Other Reactions: glycosuria, muscle spasm, weakness, restlessness, urinary bladder spasm, thrombophlebitis, transient injection site pain following intramuscular injection, fever.
-
7 DRUG INTERACTIONS
7.1 Effects of Furosemide on Other Drugs
Drug/Substance Class or Name
Drug Interaction Effect
Recommendations
Aminoglycoside antibiotics
Furosemide may increase the ototoxic potential of aminoglycoside antibiotics, especially in the presence of impaired renal function [see
Warnings and Precautions (5.3)].Avoid combination except in life-threatening situations.
Ethacrynic acid
Possibility of ototoxicity [see
Warnings and Precautions (5.3)].Avoid concomitant use with ethacrynic acid.
Salicylates
May experience salicylate toxicity at lower doses because of competitive renal excretory sites.
Monitor for symptoms of salicylate toxicity.
Cisplatin
There is a risk of ototoxic effects if cisplatin and furosemide are given concomitantly [see
Warnings and Precautions (5.3)].Cisplatin and nephrotoxic drugs
Nephrotoxicity
Administer furosemide at lower doses and with positive fluid balance when used to achieve forced diuresis during cisplatin treatment. Monitor renal function.
Paralytic agents
Furosemide has a tendency to antagonize the skeletal muscle relaxing effect of tubocurarine and may potentiate the action of succinylcholine.
Monitor for skeletal muscle effect.
Lithium
Furosemide reduces lithium's renal clearance and add a high-risk of lithium toxicity.
Avoid concomitant use with lithium.
Angiotensin converting enzyme inhibitors or angiotensin II receptor blockers
May lead to severe hypotension and deterioration in renal function, including renal failure.
Monitor for changes in blood pressure and renal function and interrupt or reduce the dosage of furosemide, angiotensin converting enzyme inhibitors, or angiotensin receptor blockers if needed.
Antihypertensive drugs
Furosemide may add to or potentiate the therapeutic effect of other antihypertensive drugs.
Monitor for changes in blood pressure and adjust the dose of other antihypertensive drugs if needed.
Adrenergic blocking drugs or peripheral adrenergic
blocking drugsPotentiation occurs.
Monitor for changes in blood pressure and adjust the dose of adrenergic blocking drugs if needed.
Norepinephrine
Furosemide may decrease arterial responsiveness (vasoconstricting effect) to norepinephrine.
Monitor blood pressure (or mean arterial pressure).
Chloral hydrate
In isolated cases, intravenous administration of furosemide within 24 hours of taking chloral hydrate may lead to flushing, sweating attacks, restlessness, nausea, increase in blood pressure, and tachycardia.
Concomitant use with chloral hydrate is not recommended.
Methotrexate and other drugs undergoing renal tubular secretion
Furosemide may decrease renal elimination of other drugs that undergo tubular secretion.
High-dose treatment of furosemide may result in elevated serum levels of these drugs and may potentiate their toxicity.Monitor serum levels of drugs undergoing renal tubular secretion and adjust the dose if needed.
Cephalosporin
Furosemide can increase the risk of cephalosporin-induced nephrotoxicity even in the setting of minor or transient renal impairment.
Monitor for changes in renal function.
Cyclosporine
Increased risk of gouty arthritis secondary to furosemide-induced hyperuricemia and cyclosporine impairment of renal urate excretion.
Monitor serum urate levels.
Thyroid hormones
High-doses (> 80 mg) of furosemide may inhibit the binding of thyroid hormones to carrier proteins and result in transient increase in free thyroid hormones, followed by an overall decrease in total thyroid hormone levels.
Monitor the total thyroid hormone levels.
7.2 Effects of Other Drugs on Furosemide
Drug/Substance Class or Name
Drug Interaction Effect
Recommendations
Phenytoin
Interferes directly with renal action of furosemide.
Monitor diuretic effects of furosemide and adjust the dose of furosemide if needed.
Methotrexate and other drugs undergoing renal tubular secretion
May reduce the effect of furosemide. High-dose treatment of methotrexate and these other drugs may result in elevated serum levels of furosemide and may potentiate the toxicity of furosemide.
Monitor for enhanced toxicity of furosemide.
Indomethacin
Coadministration of indomethacin may reduce the natriuretic and antihypertensive effects of furosemide in some patients by inhibiting prostaglandin synthesis. Indomethacin may also affect plasma renin levels, aldosterone excretion, and renin profile evaluation.
Patients receiving both indomethacin and furosemide should be observed closely to determine if the desired diuretic and/or antihypertensive effect of furosemide is achieved.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published observational studies, case reports, and postmarketing reports, from decades of use, have not demonstrated a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes with furosemide use during pregnancy. Untreated congestive heart failure and cirrhosis of the liver can lead to adverse outcomes for the mother and the fetus (see Clinical Considerations).
In animal reproduction studies, furosemide has been shown to cause unexplained maternal deaths and abortions in rabbits when administered orally during organogenesis at 4 times a human i.v. dose of 80 mg based on body surface area (BSA) and oral bioavailability corrections, presumably secondary to volume depletion (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women with congestive heart failure are at increased risk for pre-term birth. Stroke volume and heart rate increase during pregnancy, increasing cardiac output, especially during the first trimester. Clinical classification of heart disease may worsen with pregnancy and lead to maternal death and/or stillbirth. Closely monitor pregnant patients for destabilization of their heart failure.
Pregnant women with symptomatic cirrhosis generally have poor outcomes including hepatic failure, variceal hemorrhage, pre-term delivery, fetal growth restriction and maternal death. Outcomes are worse with coexisting esophageal varices. Pregnant women with cirrhosis of the liver should be carefully monitored and managed accordingly.
Data
Animal Data
The effects of furosemide on embryonic and fetal development and on pregnant dams were studied in mice, rats and rabbits.
Furosemide caused unexplained maternal deaths and abortions in the rabbit at the lowest dose of 25 mg/kg (approximately 4 times a human i.v. dose of 80 mg based on BSA and oral bioavailability corrections). In another study, a dose of 50 mg/kg (approximately 7 times a human i.v. dose of 80 mg based on BSA and oral bioavailability corrections) also caused maternal deaths and abortions when administered to rabbits between Days 12 and 17 of gestation. In a third study, none of the pregnant rabbits survived an oral dose of 100 mg/kg. Data from the above studies indicate fetal lethality that can precede maternal deaths.
The results of the mouse study and one of the three rabbit studies also showed an increased incidence and severity of hydronephrosis (distention of the renal pelvis and, in some cases, of the ureters) in fetuses of treated dams as compared with the incidence of fetuses from the control group.
8.2 Lactation
Risk Summary
The presence of furosemide has been reported in human milk. There are no data on the effects on the breastfed infant or the effects on milk production. Doses of furosemide associated with clinically significant diuresis may impair milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for furosemide and any potential adverse effects on the breastfed infant from furosemide or from the underlying maternal condition.
8.4 Pediatric Use
Published reports indicate that premature infants with post conceptual age (gestational plus postnatal) less than 31 weeks receiving doses exceeding 1 mg/kg/24 hours may develop plasma levels which could be associated with potential toxic effects including ototoxicity [see Warnings and Precautions (5.3)].
Furosemide in the first year of life, especially in patients born pre-term, may precipitate nephrocalcinosis/nephrolithiasis [see Warnings and Precautions (5.2)].
8.5 Geriatric Use
Controlled clinical studies of furosemide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for the elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
The principal signs and symptoms of overdose with furosemide are dehydration, blood volume reduction, hypotension, electrolyte imbalance, hypokalemia and hypochloremic alkalosis, and are extensions of its diuretic action.
The concentration of furosemide in biological fluids associated with toxicity or death is not known.
Treatment of overdosage is supportive and consists of replacement of excessive fluid and electrolyte losses. Serum electrolytes, carbon dioxide level, and blood pressure should be determined frequently. Adequate drainage must be assured in patients with urinary bladder outlet obstruction (such as prostatic hypertrophy).
Hemodialysis does not accelerate furosemide elimination.
-
11 DESCRIPTION
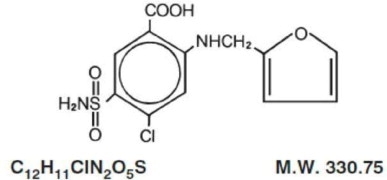
Furosemide is a diuretic which is an anthranilic acid derivative.
Chemically, it is 4-chloro-N-furfuryl-5- sulfamoylanthranilic acid.
Furosemide is a white to slightly yellow, odorless, crystalline powder. Practically insoluble in water; freely soluble in acetone, in dimethylformamide, and in solutions of alkali hydroxides; soluble in methanol; sparingly soluble in alcohol; slightly soluble in ether; very slightly soluble in chloroform. The structural formula is as follows:
Furosemide Injection, USP is a sterile, nonpyrogenic solution of furosemide in Water for Injection prepared with the aid of sodium hydroxide for intramuscular (IM) or intravenous (IV) use. Each mL contains: Furosemide 10 mg; sodium hydroxide 1.6 mg; Water for Injection q.s.; sodium chloride to adjust isotonicity; hydrochloric acid (q.s.) and/or sodium hydroxide (q.s.) to adjust pH between 8.0 and 9.3 if necessary.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Furosemide inhibits primarily the reabsorption of sodium and chloride not only in the proximal and distal tubules but also in the loop of Henle. The high degree of efficacy is largely due to this unique site of action. The action on the distal tubule is independent of any inhibitory effect on carbonic anhydrase and aldosterone.
12.2 Pharmacodynamics
The onset of diuresis following intravenous administration is within 5 minutes and somewhat later after intramuscular administration. The peak effect occurs within the first half hour. The duration of diuretic effect is approximately 2 hours.
12.3 Pharmacokinetics
Distribution
Furosemide is extensively bound to plasma proteins, mainly to albumin.
Plasma concentrations ranging from 1 to 400 mcg/mL are 91 to 99% bound in healthy individuals. The unbound fraction averages 2.3 to 4.1% at therapeutic concentrations.
Elimination
The terminal half-life of furosemide is approximately 2 hours.
Specific Populations
Geriatric Patients
Furosemide binding to albumin may be reduced in elderly patients. Furosemide is predominantly excreted unchanged in the urine. The renal clearance of furosemide after intravenous administration in older healthy male subjects (60 to 70 years of age) is statistically significantly smaller than in younger healthy male subjects (20 to 35 years of age). The initial diuretic effect of furosemide in older subjects is decreased relative to younger subjects [see Use in Specific Populations (8.5)].
Patients with Renal Impairment
One study in six subjects demonstrated that the combination of furosemide and acetylsalicylic acid temporarily reduced creatinine clearance in patients with chronic renal insufficiency. There are case reports of patients who developed increased BUN, serum creatinine and serum potassium levels, and weight gain when furosemide was used in conjunction with NSAIDs.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Furosemide was tested for carcinogenicity by oral administration in one strain of mice and one strain of rats. A small but significantly increased incidence of mammary gland carcinomas occurred in female mice at a dose approximately 8 times a human i.v. dose of 80 mg based on BSA and oral bioavailability corrections. There were marginal increases in uncommon tumors in male rats at a dose of 15 mg/kg but not at 30 mg/kg.
Mutagenesis
Furosemide was devoid of mutagenic activity in various strains of Salmonella typhimurium when tested in the presence or absence of an in vitro metabolic activation system, and questionably positive for gene mutation in mouse lymphoma cells in the presence of rat liver S9 at the highest dose tested. Furosemide did not induce sister chromatid exchange in human cells in vitro, but other studies on chromosomal aberrations in human cells in vitro gave conflicting results. In Chinese hamster cells it induced chromosomal damage but was questionably positive for sister chromatid exchange. Studies on the induction by furosemide of chromosomal aberrations in mice were inconclusive. The urine of rats treated with this drug did not induce gene conversion in Saccharomyces cerevisiae.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Store at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature.] Protect from light.
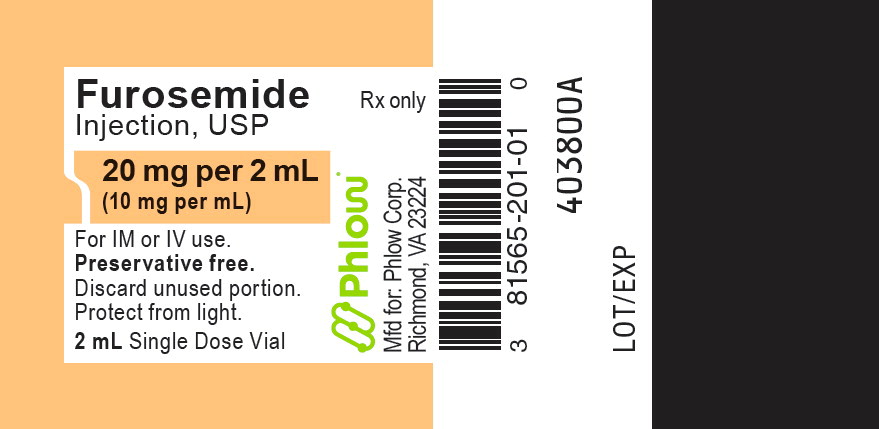
Furosemide Injection, USP is a sterile, colorless solution for injection, available as a single-dose vial that contains 10 mg/mL of furosemide, and is supplied as follows:
Product Code
PC28002
Unit of Sale
NDC: 81565-201-02
Unit of 25Strength
20 mg per 2 mL
(10 mg per mL)Each
NDC: 81565-201-01
2 mL in a 2 mL single dose amber vialPreservative Free.
Discard unused portion. Do not use if solution is discolored or contains particulate.
-
17 PATIENT COUNSELING INFORMATION
Fluid, Electrolyte, and Metabolic Abnormalities
Advise patients that they may experience symptoms from excessive fluid and/or electrolyte losses. The postural hypotension that sometimes occurs can usually be managed by getting up slowly. Potassium supplements and/or dietary measures may be needed to control or avoid hypokalemia [see Warnings and Precautions (5.1)].
Advise patients that furosemide may increase blood glucose levels and thereby affect urine glucose tests [see Warnings and Precautions (5.1)].
Photosensitivity
The skin of some patients may be more sensitive to the effects of sunlight while taking furosemide [see Adverse Reactions (6)].
Advise hypertensive patients to avoid medications that may increase blood pressure, including over-the-counter products for appetite suppression and cold symptoms [see Drug Interactions (7.1)].
For more information concerning this drug, please call Fresenius Kabi USA, LLC at 1-800-551-7176.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Mfd. for:
Richmond, VA 23224
www.Phlow-USA.comMfd. by:
Fresenius Kabi
Lake Zurich, IL 60047451725A
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY - Furosemide 2 mL Single Dose Vial Tray Label
NDC: 81565-201-02 PC28002
Furosemide Injection, USP
20 mg per 2 mL (10 mg per mL)
For intramuscular or intravenous use.
Preservative free.25 x 2 mL
Single Dose Vials Rx only -
INGREDIENTS AND APPEARANCE
FUROSEMIDE
furosemide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 81565-201 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FUROSEMIDE (UNII: 7LXU5N7ZO5) (FUROSEMIDE - UNII:7LXU5N7ZO5) FUROSEMIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81565-201-02 25 in 1 TRAY 02/01/2022 1 NDC: 81565-201-01 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018902 02/01/2022 Labeler - Phlow Corporation (117445813) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 840771732 MANUFACTURE(81565-201) , ANALYSIS(81565-201) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 023648251 ANALYSIS(81565-201) , MANUFACTURE(81565-201)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.