SODIUM CHLORIDE injection
SODIUM CHLORIDE by
Drug Labeling and Warnings
SODIUM CHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
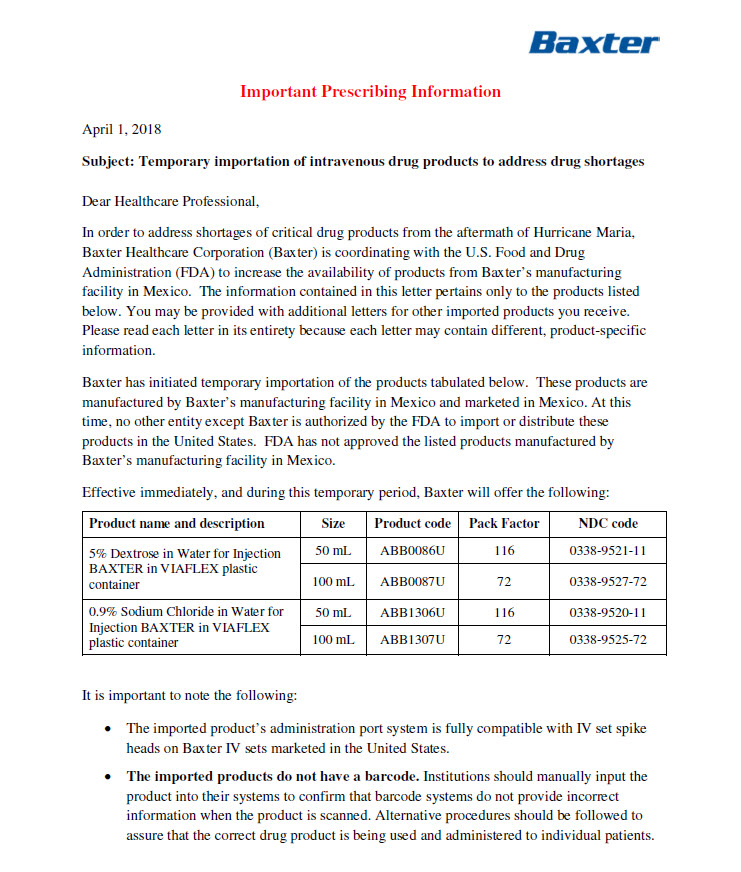
- HEALTH CARE PROVIDER LETTER
-
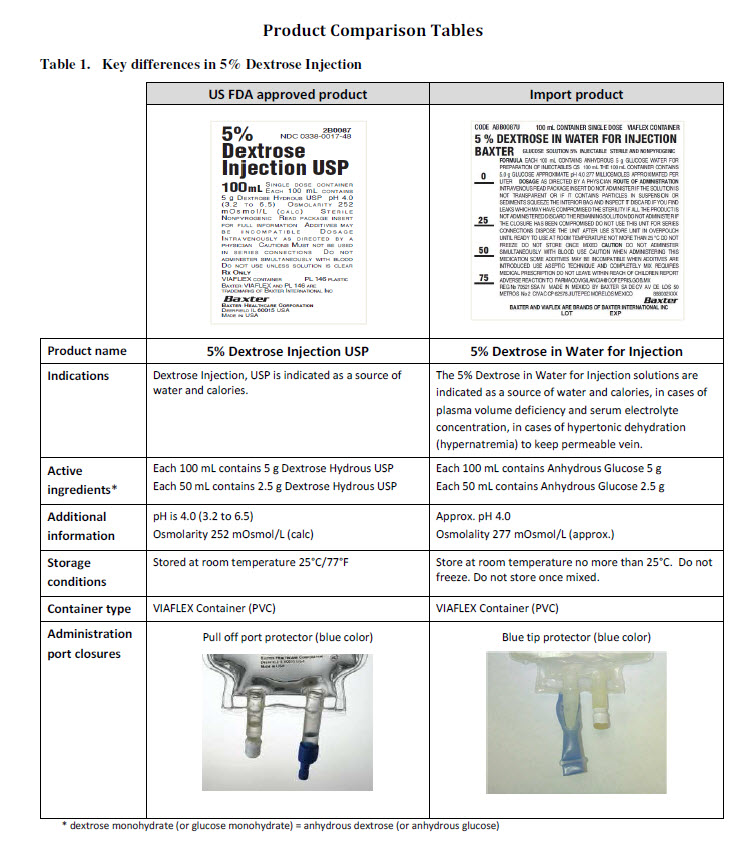
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
CODE ABB1306U
50 mL CONTAINER SINGLE DOSE
0.9% SODIUM CHLORIDE IN WATER
SODUIM CHLORIDE FOR INJECTION BAXTER
VIAFLEX CONTAINER
MINI-BAG
SOLUTION 0.9% INJECTABLEFORMULA EACH 100 mL CONTAINS 0.9 g SODIUM CHLORIDE WATER FOR PREPARATION
OF INJECTABLES QS 100 mL APPROXIMATE pH 5 THE 50 mL CONTAINER CONTAINS 7.7
mEq SODIUM 7.7 mEq CHLORIDE 308 MILLIOSMOLES APPROXIMATED PER LITER
DOSAGE AS DIRECTED BY A PHYSICIAN ROUTE OF ADMINISTRATION INTRAVENOUS
READ PACKAGE INSERT DO NOT ADMINISTER IF THE SOLUTION IS NOT TRANSPARENT
OR IF IT CONTAINS PARTICLES IN SUSPENSION OR SEDIMENTS SQUEEZE THE INTERIOR
BAG AND INSPECT IT DISCARD IF YOU FIND LEAKS WHICH MAY HAVE COMPROMISED
THE STERILITY IF ALL THE PRODUCT IS NOT ADMINISTERED DISCARD THE REMAINING
SOLUTION DO NOT ADMINISTER IF THE CLOSURE HAS BEEN COMPROMISED DO NOT
USE THIS UNIT FOR SERIES CONNECTIONS DISPOSE THE UNIT AFTER USE STORE UNIT
IN OVERPOUCH UNTIL READY TO USE AT ROOM TEMPERATURE NOT MORE THAN 25 °C
DO NOT STORE ONCE MIXED CAUTION DO NOT ADMINISTER SIMULTANEOUSLY WITH
BLOOD REQUIRES MEDICAL PRESCRIPTION DO NOT LEAVE WITHIN REACH OF
CHILDREN SOME DRUGS MAY BE INCOMPATIBLE WHEN DRUGS ARE INTRODUCED USE
ASEPTlC TECHNIQUES AND COMPLETELY MIX REPORT ADVERSE REACTION TO
FARMACOVIGILANCIA@COFEPRIS.GOB.MX REG No 70508 SSA IV MADE IN MEXICO BY BAXTER SA DE CV AV DE LOS 50 METROS No 2 CIVAC CP 62578 JIUTEPEC MORELOS
MEXICO
88 80 32 1000Baxter Logo
BAXTER MINI-BAG AND VIAFLEX ARE BRANDS OF BAXTER INTERNATIONAL INCLOT EXP
0
25
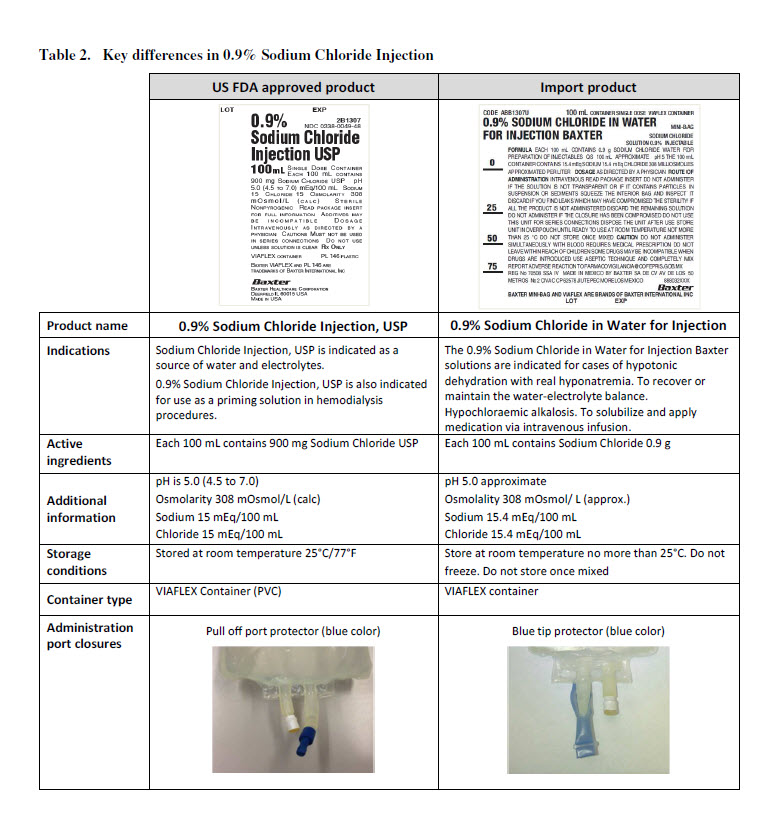
CODE ABB1307U
100 mL CONTAINER SINGLE DOSE
0.9% SODIUM CHLORIDE IN WATER
FOR INJECTION BAXTER
VIAFLEX CONTAINER
MINI-BAG
SODIUM CHLORIDE
SOLUTION 0.09% INJECTABLEFORMULA EACH 100 mL CONTAINS 0.9 g SODIUM CHLORIDE WATER FOR
PREPARATION OF INJECTABLES QS 100 mL APPROXIMATE pH 5 THE 100 Ml
CONTAINER CONTAINS 15.4 mEq SODIUM 15.4 mEq CHLORIDE 308 MILLIOSMOLES
APPROXIMATED PER LITER DOSAGE AS DIRECTED BY A PHYSICIAN ROUTE OF ADMINISTRATION INTRAVENOUS READ PACKAGE INSERT SO NOT ADMINISTER
IF THE SOLUTION IS NOT TRANSPARENT OR IF IT CONTAINS PARTICLES IN
SUSPENSION OR SEDIMENTS SQUEEZE THE INTERIOR BAG AND INSPECT IT
DISCARD IF YOU FIND LEAKS WHICH MAY HAVE COMPROMISED THE STERILITY IF
ALL THE PROOUCT IS NOT ADMINISTERED DISCARD THE REMAINING SOLUTION
DO NOT ADMINISTER IF THE CLOSURE HAS BEEN COMPROMISED DO NOT USE
THIS UNIT FOR SERIES CONNECTIONS DISPOSE THE UNIT AFTER USE STORE UNIT IN OVERPOUCH UNTIL READY TO USE AT ROOM TEMPERATURE NOT MORE
THAN 25 °C DO NOT STORE ONCE MIXED CAUTION DO NOT ADMINISTER
SIMULTANEOUSLY WITH BLOOD REQUIRES MEDICAL PRESCRIPTION DO NOT
LEAVE WITHIN REACH OF CHILDREN SOME DRUGS MAYBE INCOMPATIBLE WHEN
DRUGS ARE INTRODUCED USE ASEPTIC TECHNIQUE AND COMPLETELY MIX
REPORT ADVERSE REACTION TO FARMACOVIGILANCIA@COFEPRIS.GOB.MX
REG No 70508 SSA IV MADE IN MEXICO BY BAXTER SA DE CV AV DE LOS 50
METROS No 2 CIVAC CP 62578 JIUTEPEC MORELOS MEXICO
88 80 32 999Baxter Logo
BAXTER MINI-BAG AND VIAFLEX ARE BRANDS OF BAXTER INTERNATIONAL INCLOT EXP
0
25
50
75
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9520 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.9 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9520-11 116 in 1 CARTON 10/19/2017 10/19/2017 1 50 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/19/2017 10/19/2017 SODIUM CHLORIDE
sodium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9525 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.9 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9525-72 72 in 1 CARTON 10/19/2017 1 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/19/2017 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter, S.A. de C.V. 810432484 ANALYSIS(0338-9525) , LABEL(0338-9525) , MANUFACTURE(0338-9525) , PACK(0338-9525) , STERILIZE(0338-9525)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.