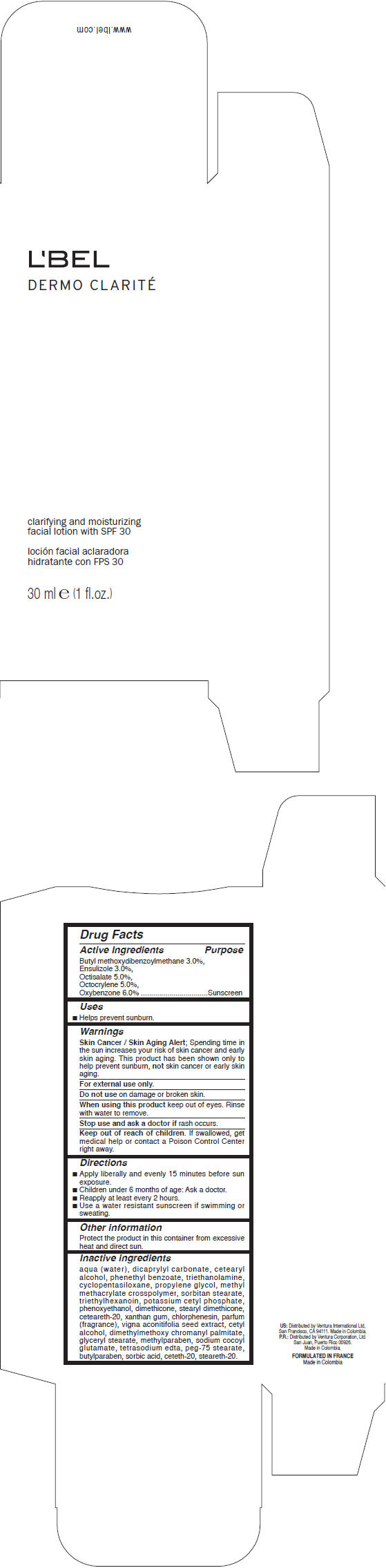
LBEL DERMO CLARITE CLARIFYING AND MOISTURIZING WITH SPF 30- avobenzone, ensulizole, octisalate, octocrylene, and oxybenzone lotion
LBEL by
Drug Labeling and Warnings
LBEL by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation Ltd., Bel Star, S. A.(Colombia), Yobel Supply Chain Management (Peru). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
water, dicaprylyl carbonate, cetearyl alcohol, phenethyl benzoate, triethanolamine, cyclopentasiloxane, propylene glycol, methyl methacrylate crosspolymer, sorbitan stearate, triethylhexanoin, potassium cetyl phosphate, phenoxyethanol, dimethicone, stearyl dimethicone, ceteareth-20, xanthan gum, chlorphenesin, fragrance, vigna aconitifolia seed extract, cetyl alcohol, dimethylmethoxy chromanyl palmitate, glyceryl stearate, methylparaben, sodium cocoyl glutamate, tetrasodium edta, peg-75 stearate, butylparaben, sorbic acid, ceteth-20, steareth-20.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
LBEL DERMO CLARITE CLARIFYING AND MOISTURIZING WITH SPF 30
avobenzone, ensulizole, octisalate, octocrylene, and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-404 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 0.03 g in 1 mL Ensulizole (UNII: 9YQ9DI1W42) (Ensulizole - UNII:9YQ9DI1W42) Ensulizole 0.03 g in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.05 g in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.05 g in 1 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.06 g in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) phenethyl benzoate (UNII: 0C143929GK) trolamine (UNII: 9O3K93S3TK) cyclomethicone 5 (UNII: 0THT5PCI0R) propylene glycol (UNII: 6DC9Q167V3) sorbitan monostearate (UNII: NVZ4I0H58X) triethylhexanoin (UNII: 7K3W1BIU6K) potassium cetyl phosphate (UNII: 03KCY6P7UT) phenoxyethanol (UNII: HIE492ZZ3T) dimethicone (UNII: 92RU3N3Y1O) polyoxyl 20 cetostearyl ether (UNII: YRC528SWUY) xanthan gum (UNII: TTV12P4NEE) chlorphenesin (UNII: I670DAL4SZ) moth bean (UNII: H7938ON8E5) cetyl alcohol (UNII: 936JST6JCN) glyceryl monostearate (UNII: 230OU9XXE4) methylparaben (UNII: A2I8C7HI9T) edetate sodium (UNII: MP1J8420LU) peg-75 stearate (UNII: OT38R0N74H) butylparaben (UNII: 3QPI1U3FV8) sorbic acid (UNII: X045WJ989B) ceteth-20 (UNII: I835H2IHHX) steareth-20 (UNII: L0Q8IK9E08) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-404-01 1 in 1 BOX 1 NDC: 13537-404-02 30 mL in 1 BOTTLE 2 NDC: 13537-404-03 1 in 1 BOX 2 NDC: 13537-404-04 3 mL in 1 BOTTLE 3 NDC: 13537-404-05 1 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/13/2011 Labeler - Ventura Corporation Ltd. (602751344) Establishment Name Address ID/FEI Business Operations Bel Star, S. A.(Colombia) 880160197 MANUFACTURE(13537-404) Establishment Name Address ID/FEI Business Operations Yobel Supply Chain Management (Peru) 934116930 MANUFACTURE(13537-404)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.