OBAGI SKINTRINSIQ CLARIFIYING PROTOCOL- salicylic acid, titanium dioxide, and zinc oxide kit
Obagi Skintrinsiq Clarifiying Protocol by
Drug Labeling and Warnings
Obagi Skintrinsiq Clarifiying Protocol by is a Otc medication manufactured, distributed, or labeled by Obagi Cosmeceuticals LLC, G.S.COSMECEUTICAL USA, INC., Denison Pharmaceuticals, LLC, Swiss-American CDMO, LLC, TENCO ASSEMBLIES, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Cleanse the skin thoroughly before applying this product.
- Shake product well before use.
- Cover the entire affected area with a thin layer one to three times daily. Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive ingredients
Water (Aqua), Caprylic/Capric Triglyceride, Dicaprylyl Carbonate, Pentylene Glycol, Steareth-2, Glycerin, Silica, Polyhydroxystearic Acid, Steareth-21, Cetearyl Alcohol, Propanediol, Alumina, Stearic Acid, Sodium Stearoyl Glutamate, Caprylyl Glycol, Triethoxycaprylylsilane, Cetearyl Glucoslde, Xanthan Gum, Dipotassium Glycyrrhizate,Trisodium Ethylenediamine Disuccinate, Caprylhydroxamic Acid, Tropaeolum Majus Flower/Leaf/Stem Extract
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
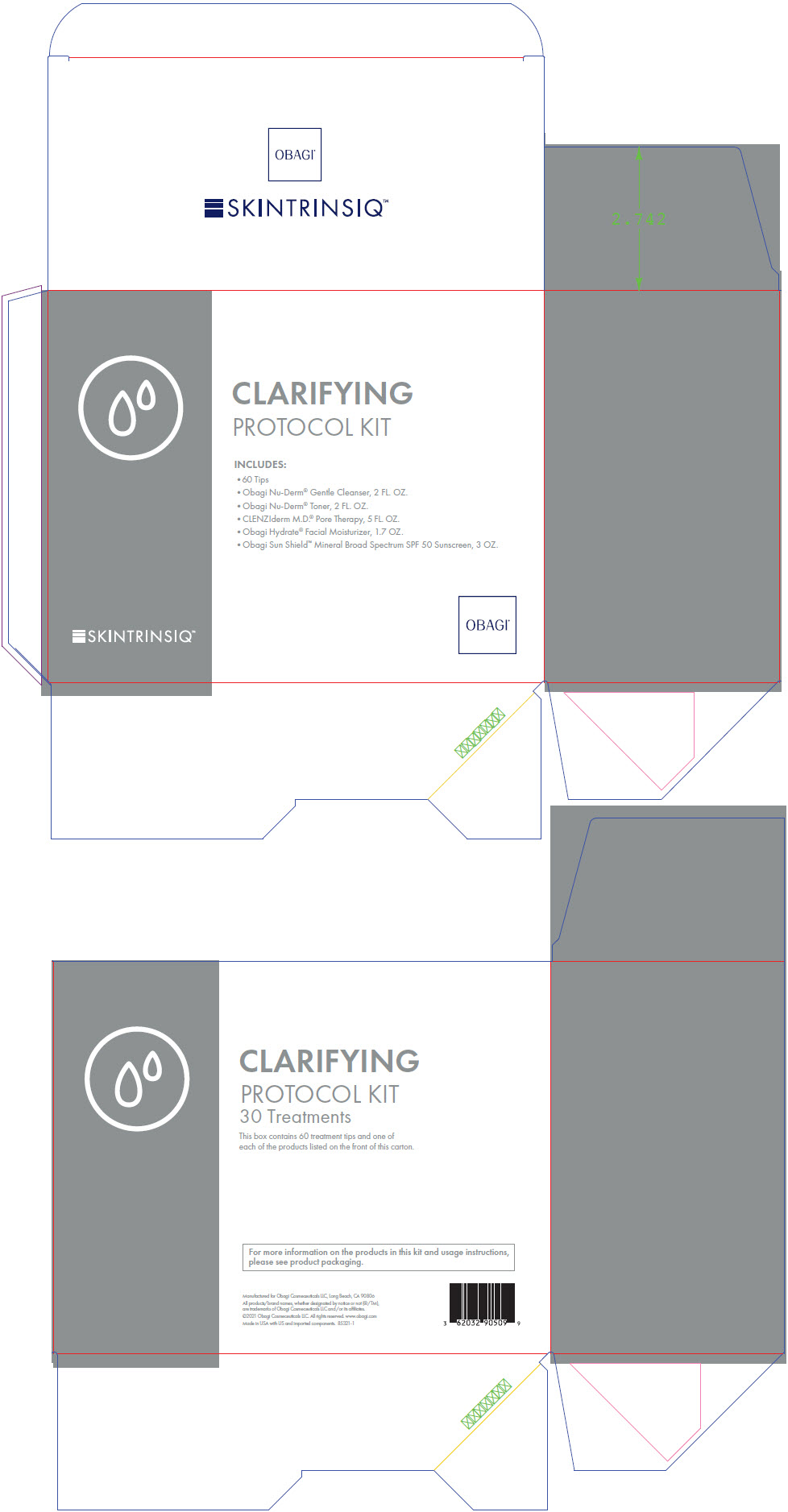
- PRINCIPAL DISPLAY PANEL - Kit Carton
- PRINCIPAL DISPLAY PANEL - 148 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
-
INGREDIENTS AND APPEARANCE
OBAGI SKINTRINSIQ CLARIFIYING PROTOCOL
salicylic acid, titanium dioxide, and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62032-909 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-909-09 1 in 1 BOX 08/01/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 60 mL Part 2 1 BOTTLE, PLASTIC 59 mL Part 3 1 BOTTLE, PLASTIC 148 mL Part 4 1 BOTTLE, PUMP 48 g Part 5 1 TUBE 85 g Part 1 of 5 OBAGI NU-DERM GENTLE CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) [skin care preparations (creams, lotions, powder, and sprays)] gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR APRICOT KERNEL OIL (UNII: 54JB35T06A) INGR BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) INGR BUTYLPARABEN (UNII: 3QPI1U3FV8) INGR CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) INGR ETHYLPARABEN (UNII: 14255EXE39) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR GLYCERETH-7 (UNII: 3D2Y91QZ2H) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ISOBUTYLPARABEN (UNII: 0QQJ25X58G) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR OLEYL LACTATE (UNII: B3AWW0N3GM) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR SAGE (UNII: 065C5D077J) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR SODIUM LAUROYL OAT AMINO ACIDS (UNII: FSW2K9B9N5) INGR TROLAMINE (UNII: 9O3K93S3TK) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/01/2021 Part 2 of 5 OBAGI NU-DERM TONER
face and neck (excluding shaving preparations), leave-on [skin care preparations (creams, lotions, powder, and sprays)] liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ALLANTOIN (UNII: 344S277G0Z) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) INGR CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM ALUM (UNII: 1L24V9R23S) INGR SAGE (UNII: 065C5D077J) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/01/2021 Part 3 of 5 CLENZIDERM PORE THERAPY ACNE TREATMENT
salicylic acid liquidProduct Information Item Code (Source) NDC: 62032-612 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) CHAMOMILE (UNII: FGL3685T2X) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) WATER (UNII: 059QF0KO0R) Product Characteristics Color purple Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-612-04 148 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M006 08/01/2021 Part 4 of 5 OBAGI HYDRATE FACIAL MOISTURIZER
moisturizing [skin care preparations (creams, lotions, powder, and sprays)] lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ALLANTOIN (UNII: 344S277G0Z) INGR AVOCADO OIL (UNII: 6VNO72PFC1) INGR CAESALPINIA SPINOSA RESIN (UNII: WL3883U2PO) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR LAURETH-12 (UNII: OAH19558U1) INGR LEVOMENOL (UNII: 24WE03BX2T) INGR MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SACCHARIDE ISOMERATE (UNII: W8K377W98I) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) INGR SOY STEROL (UNII: PL360EPO9J) INGR SOYBEAN (UNII: L7HT8F1ZOD) INGR STEARIC ACID (UNII: 4ELV7Z65AP) INGR TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 48 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/01/2021 Part 5 of 5 SUN SHIELD MINERAL BROAD SPECTRUM SPF 50 SUNSCREEN
titanium dioxide and zinc oxide lotionProduct Information Item Code (Source) NDC: 62032-811 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 6.2 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 15.6 g in 100 g Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PROPANEDIOL (UNII: 5965N8W85T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) TROPAEOLUM MAJUS FLOWERING TOP (UNII: RGT30824HY) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-811-09 1 in 1 CARTON 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/01/2021 Labeler - Obagi Cosmeceuticals LLC (790553353) Establishment Name Address ID/FEI Business Operations G.S.COSMECEUTICAL USA, INC. 017014734 manufacture(62032-909) Establishment Name Address ID/FEI Business Operations Denison Pharmaceuticals, LLC 001207208 manufacture(62032-909) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(62032-909) Establishment Name Address ID/FEI Business Operations TENCO ASSEMBLIES, INC 075535609 pack(62032-909)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.