Triple Antibiotic Plus Pain Relief
Galentic Triple Antibiotic Plus Pain Relief by
Drug Labeling and Warnings
Galentic Triple Antibiotic Plus Pain Relief by is a Otc medication manufactured, distributed, or labeled by Galentic Pharma (India) Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
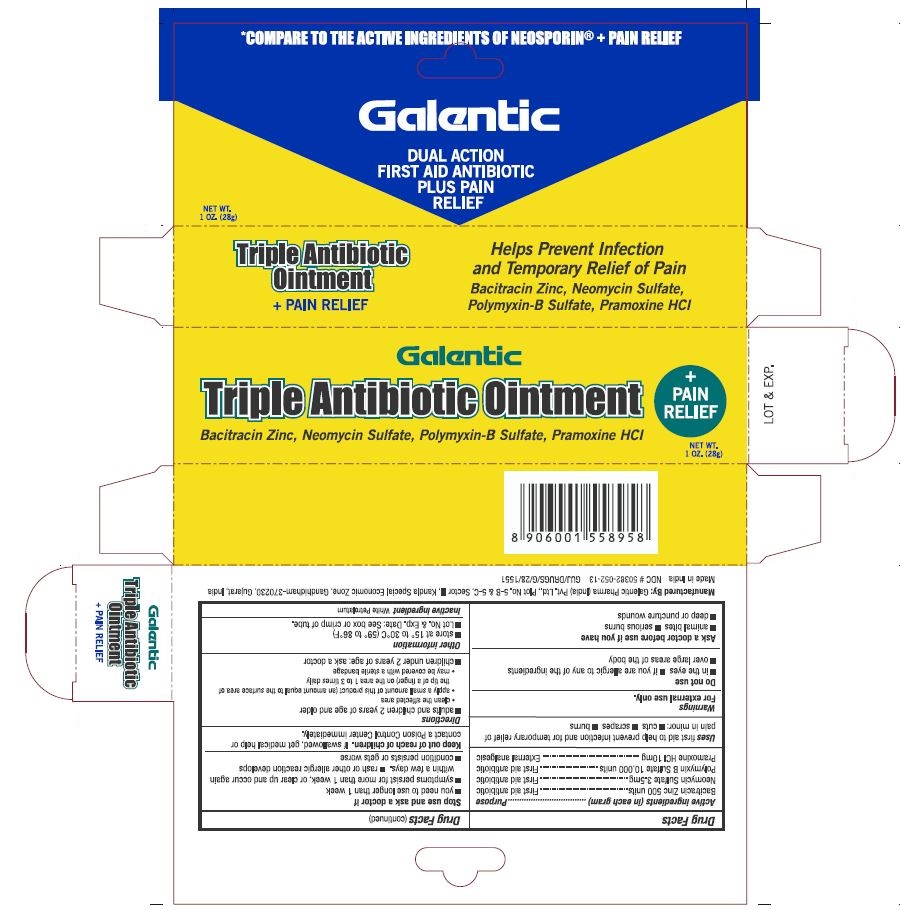
GALENTIC TRIPLE ANTIBIOTIC PLUS PAIN RELIEF- bacitracin zinc, neomycin sulfate, polymyxin b sulfate, pramoxine hcl ointment
Galentic Pharma (India) Private Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Triple Antibiotic Plus Pain Relief
Active Ingredients (in each gram)
Bacitracin Zinc 500 units
Neomycin Sulfate 3.5 mg
Polymyxin B Sulfate 10,000 units
Pramoxine HCl 10mg
First aid antibiotic (Bacitracin Zinc 500 units, Neomycin Sulfate 3.5 mg, & Polymyxin B Sulfate 10,000 units)
External Analgesic (Pramoxine HCl 10mg)
First aid to help prevent infection and for temporary relief of pain in minor cuts, scrapes and burns
For external use only
Do not use
In the eyes
If you are allergic to any of the ingredients
Over large areas of the body
Ask a doctor before use if you have
Animal bites
Serious Burns
Deep or puncture wounds
Stop use and ask a doctor if
You need to use longer than 1 week
condition persists or gets worse
rash or other allergic reaction develops
symptoms persist for more than 1 week, or clear up and occur again within a few days
| GALENTIC TRIPLE ANTIBIOTIC PLUS PAIN RELIEF
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, pramoxine hcl ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Galentic Pharma (India) Private Limited (915110464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Galentic Pharma (India) Private Limited | 650970176 | manufacture(50382-052) , analysis(50382-052) , pack(50382-052) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.