MUCINEX by A-S Medication Solutions Mucinex®
MUCINEX by
Drug Labeling and Warnings
MUCINEX by is a Otc medication manufactured, distributed, or labeled by A-S Medication Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
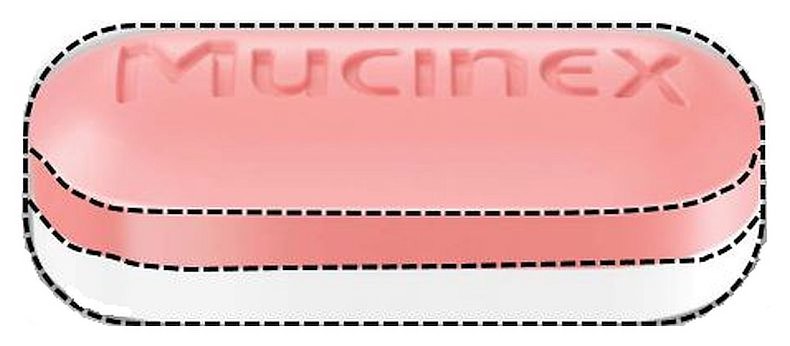
MUCINEX- guaifenesin tablet, extended release
A-S Medication Solutions
----------
Mucinex®
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- children under 12 years of age: do not use
| MUCINEX
guaifenesin tablet, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - A-S Medication Solutions (830016429) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| A-S Medication Solutions | 830016429 | RELABEL(50090-2380) , REPACK(50090-2380) | |
Revised: 1/2020
Document Id: 866f977d-aef5-4dd5-8c11-b264d8631cb9
Set id: 08ea92cc-4a0f-44c9-9edf-71f67e014431
Version: 9
Effective Time: 20200118
Trademark Results [MUCINEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCINEX 98499792 not registered Live/Pending |
RB Health (US) LLC 2024-04-15 |
 MUCINEX 90229382 not registered Live/Pending |
RB Health (US) LLC 2020-10-01 |
 MUCINEX 88040437 not registered Live/Pending |
Reckitt Benckiser LLC 2018-07-17 |
 MUCINEX 86191608 4613098 Live/Registered |
RB HEALTH (US) LLC 2014-02-12 |
 MUCINEX 85496330 4168070 Live/Registered |
RB HEALTH (US) LLC 2011-12-15 |
 MUCINEX 85489681 4168052 Live/Registered |
RB HEALTH (US) LLC 2011-12-07 |
 MUCINEX 77770135 3722363 Live/Registered |
Reckitt Benckiser Inc. 2009-06-29 |
 MUCINEX 76297961 2670161 Live/Registered |
RB HEALTH (US) LLC 2001-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
