TEVIMBRA- tislelizumab-jsgr injection, solution, concentrate
TEVIMBRA by
Drug Labeling and Warnings
TEVIMBRA by is a Prescription medication manufactured, distributed, or labeled by BeOne Medicines USA, Inc., Boehringer Ingelheim Biopharmaceuticals (China) Ltd., BeOne Pharmaceutical (Suzhou) Co.,Ltd, Sharp Packaging Services, LLC, Microcoat Biotechnologie GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TEVIMBRA safely and effectively. See full prescribing information for TEVIMBRA.
TEVIMBRA® (tislelizumab-jsgr) injection, for intravenous use
Initial U.S. Approval: 2024RECENT MAJOR CHANGES
INDICATIONS AND USAGE
TEVIMBRA is a programmed death receptor-1 (PD-1)-blocking antibody indicated for:
Esophageal Cancer
- in combination with platinum-containing chemotherapy for the first-line treatment of adults with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors express PD-L1 (≥1). (1.1)
- as a single agent in adults with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) after prior systemic chemotherapy that did not include a PD-(L)1 inhibitor. (1.1)
Gastric Cancer
- in combination with platinum and fluoropyrimidine-based chemotherapy in adults for the first line treatment of unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (≥1). (1.2)
DOSAGE AND ADMINISTRATION
Recommended Dosage:
Esophageal Cancer
- 150 mg every 2 weeks or 200 mg every 3 weeks or 300 mg every 4 weeks or 400 mg every 6 weeks in combination with platinum-containing chemotherapy for first-line treatment of unresectable or metastatic ESCC. (2.2)
- 150 mg every 2 weeks or 200 mg every 3 weeks or 300 mg every 4 weeks or 400 mg every 6 weeks as a single agent for treatment of unresectable or metastatic ESCC. (2.2)
Gastric Cancer
- 150 mg every 2 weeks or 200 mg every 3 weeks or 300 mg every 4 weeks or 400 mg every 6 weeks in combination with platinum and fluoropyrimidine-based chemotherapy. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/10 mL (10 mg/mL) solution in a single-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
-
Immune-Mediated Adverse Reactions: (5.1)
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions, and solid organ transplant rejection.
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment.
- Withhold or permanently discontinue TEVIMBRA based on the severity of reaction.
- Infusion-Related Reactions: Slow the rate of infusion, interrupt, or permanently discontinue based on severity of infusion reaction. (5.2)
- Complications of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT): Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. (5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (≥20%), including laboratory abnormalities, were:
- TEVIMBRA in combination with platinum-containing chemotherapy: decreased neutrophil count, decreased sodium, increased glucose, anemia, fatigue, decreased appetite, increased AST, decreased potassium, increased serum creatinine, decreased calcium, increased ALT, diarrhea, stomatitis, and vomiting. (6.1)
- TEVIMBRA as a single agent: increased glucose, decreased hemoglobin, decreased lymphocytes, decreased sodium, decreased albumin, increased alkaline phosphatase, anemia, fatigue, increased AST, musculoskeletal pain, decreased weight, increased ALT, and cough. (6.1)
- TEVIMBRA in combination with platinum and fluoropyrimidine-based chemotherapy: nausea, fatigue, decreased appetite, anemia, peripheral sensory neuropathy, vomiting, decreased platelet count, decreased neutrophil count, increased aspartate aminotransferase, diarrhea, abdominal pain, increased alanine aminotransferase, decreased white blood cell count, decreased weight, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BeOne Medicines at 1-877-828-5596 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Esophageal Cancer
1.2 Gastric Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Dosage Modifications for Adverse Reactions
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Infusion-Related Reactions
5.3 Complications of Allogeneic HSCT
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Esophageal Squamous Cell Carcinoma
14.2 Gastric Cancer
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Esophageal Cancer
- First-Line Treatment of Esophageal Squamous Cell Carcinoma
TEVIMBRA, in combination with platinum-containing chemotherapy, is indicated for the first-line treatment of adults with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors express PD-L1 (≥1).
- Previously Treated Esophageal Squamous Cell Carcinoma
TEVIMBRA, as a single agent, is indicated for the treatment of adults with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) after prior systemic chemotherapy that did not include a PD-(L)1 inhibitor.
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the first-line treatment of unresectable or metastatic esophageal squamous cell carcinoma based on the presence of PD-L1 in tumor specimens [see Clinical Studies (14.1)]. An FDA-approved companion diagnostic for the detection of PD-L1 in patients with unresectable or metastatic esophageal squamous cell carcinoma is not available.
Select patients for the first-line treatment of unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma (G/GEJ) based on the presence of PD-L1 in tumor specimens [see Clinical Studies (14.2)]. An FDA-approved companion diagnostic for the detection of PD-L1 in patients with unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma (G/GEJ) is not available.
2.2 Recommended Dosage
The recommended dosages of TEVIMBRA administered intravenously as a single agent or in combination with other therapeutic agents are presented in Table 1.
Table 1: Recommended Dosages for TEVIMBRA as a Single Agent or in Combination with Other Therapeutic Agents Indication Recommended Dosage of TEVIMBRA Duration/Timing of Treatment ESCC
OR
First-Line Gastric Cancer150 mg every 2 weeks
OR
200 mg every 3 weeks
OR
300 mg every 4 weeks
OR
400 mg every 6 weeksUntil disease progression or unacceptable toxicity. Refer to the respective Prescribing Information for each therapeutic agent administered in combination with TEVIMBRA for the recommended dosage information, as appropriate.
2.3 Dosage Modifications for Adverse Reactions
No dose reduction of TEVIMBRA is recommended. In general, withhold TEVIMBRA for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue TEVIMBRA for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone equivalent per day within 12 weeks of initiating steroids [see Warnings and Precautions (5.1)].
Dosage modifications for TEVIMBRA for adverse reactions that require management different from these general guidelines are summarized in Table 2.
Refer to the respective Prescribing Information for dosage modifications for the platinum and fluoropyrimidine agent administered in combination with TEVIMBRA.
Table 2: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity of Adverse Reaction* Dosage Modifications ALT = alanine aminotransferase, AST = aspartate aminotransferase, ULN = upper limit of normal, SJS = Stevens-Johnson syndrome, TEN = toxic epidermal necrolysis, DRESS = drug rash with eosinophilia and systemic symptoms. - * Based on Common Terminology Criteria for Adverse Events (CTCAE) Version 4.
- † Resume in patients with complete or partial resolution (Grades 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating steroids or inability to reduce prednisone to 10 mg per day or less (or equivalent) within 12 weeks of initiating steroids.
- ‡ If AST and ALT are less than or equal to ULN at baseline, withhold or permanently discontinue TEVIMBRA based on recommendations for hepatitis with no liver involvement.
- § Resume infusion if resolved or decreased to Grade 1, and slow rate of infusion by 50% of the previous rate.
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] Pneumonitis Grade 2 Withhold† Grade 3 or 4 or recurrent Grade 2 Permanently discontinue Colitis Grade 2 or 3 Withhold† Grade 4 Permanently discontinue Hepatitis with no tumor involvement of the liver AST or ALT increases to more than 3 and up to 8 times ULN
or
Total bilirubin increases to more than 1.5 and up to 3 times ULNWithhold† AST or ALT increases to more than 8 times ULN
or
Total bilirubin increases to more than 3 times ULNPermanently discontinue Hepatitis with tumor involvement of the liver‡ Baseline AST or ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN
or
Baseline AST or ALT is more than 3 and up to 5 times ULN and increases to more than 8 and up to 10 times ULNWithhold† ALT or AST increases to more than 10 times ULN
or
Total bilirubin increases to more than 3 times ULNPermanently discontinue Endocrinopathies Grade 3 or 4 Withhold until clinically stable or permanently discontinue depending on severity Nephritis with renal dysfunction Grade 2 or 3 increased blood creatinine Withhold† Grade 4 increased blood creatinine Permanently discontinue Exfoliative dermatologic conditions Grade 3, or suspected SJS, TEN, or DRESS Withhold† Grade 4, or confirmed SJS, TEN, or DRESS Permanently discontinue Myocarditis Grade 2, 3, or 4 Permanently discontinue Neurological toxicities Grade 2 Withhold† Grade 3 or 4 Permanently discontinue Other Adverse Reactions Infusion-related reactions [see Warnings and Precautions (5.2)] Grade 1 Slow infusion rate by 50% Grade 2 Interrupt infusion§ Grade 3 or 4 Permanently discontinue 2.4 Preparation and Administration
Preparation
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. TEVIMBRA is a clear to slightly opalescent, colorless to slightly yellow solution. Discard the vial if the solution is cloudy, discolored, or contains visible particles. Do not shake the vial.
Prepare the solution for infusion as follows:
- Withdraw the required volume of TEVIMBRA from the vial(s).
- Transfer solution into an intravenous infusion bag containing 0.9% Sodium Chloride Injection, USP to prepare an infusion solution with a final concentration of 2 mg/mL to 5 mg/mL.
- Mix diluted solution by gentle inversion to avoid foaming or excessive shearing of the solution. Do not shake.
- TEVIMBRA is for single use only. Discard any unused portion left in the vial.
Storage of Diluted Solution
This product does not contain any preservatives. If not used immediately, store the TEVIMBRA diluted solution either:
- At room temperature at 20°C to 25°C (68°F to 77°F) for up to 4 hours, including preparation and infusion duration. Discard after 4 hours.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for up to 10 days (240 hours), including preparation and infusion duration. Allow the diluted solution to come to room temperature prior to administration. Discard after 10 days (240 hours).
Protect diluted solution from light during storage. Do not freeze the diluted solution.
Administration
- Administer diluted solution by intravenous infusion through an intravenous line with a sterile, nonpyrogenic, low protein binding 0.2 micron or 0.22 micron in-line or add-on filter.
- For 150 mg and 200 mg doses, administer the initial infusion over 60 minutes. If tolerated, all subsequent infusions may be administered over 30 minutes.
For 300 mg doses, administer the initial infusion over 90 minutes. If tolerated, administer the second infusion over 60 minutes. If the second infusion is tolerated, administer subsequent infusions over 30 minutes.
For 400 mg doses, administer the initial infusion over 120 minutes. If tolerated, administer the second infusion over 60 minutes. If the second infusion is tolerated, administer subsequent infusions over 30 minutes. - Do NOT coadminister other drugs through the same infusion line.
- Do NOT administer TEVIMBRA as an intravenous push or single bolus injection.
- Flush the intravenous line at the end of infusion.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
TEVIMBRA is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death receptor-1 (PD-1) or PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under WARNINGS AND PRECAUTIONS may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue TEVIMBRA depending on severity [see Dosage and Administration (2.2)]. In general, if TEVIMBRA requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroids.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
TEVIMBRA can cause immune-mediated pneumonitis, which can be fatal. In patients treated with other PD-1/PD-L1 blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Immune-mediated pneumonitis occurred in 4.7% (113/2390) of patients receiving TEVIMBRA, including fatal (0.1%), Grade 4 (0.3%), Grade 3 (1.4%), and Grade 2 (1.9%) adverse reactions. Pneumonitis led to permanent discontinuation of TEVIMBRA in 44 (1.8%) patients and withholding of TEVIMBRA in 40 (1.7%) patients.
Eighty-one (71.7%) of the 113 patients received systemic corticosteroids. Seventy-four (65.5%) of the 113 patients received high-dose systemic corticosteroids. Immune-mediated pneumonitis resolved in 48.7% of the 113 patients. Of the 40 patients in whom TEVIMBRA was withheld for pneumonitis, 26 (65%) reinitiated TEVIMBRA after symptom improvement; of these, 5 (19%) patients had recurrence of pneumonitis.
Immune-Mediated Colitis
TEVIMBRA can cause immune-mediated colitis, which can be fatal. Cytomegalovirus infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis treated with PD-1/PD-L1 blocking antibodies. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Immune-mediated colitis occurred in 0.8% (19/2390) of patients receiving TEVIMBRA, including Grade 3 (0.3%) and Grade 2 (0.4%) adverse reactions. Colitis led to permanent discontinuation of TEVIMBRA in 5 (0.2%) patients and withholding of TEVIMBRA in 10 (0.4%) patients. Seventeen (89.5%) of the 19 patients received systemic corticosteroids. Twelve (63.2%) of the 19 patients received high-dose systemic corticosteroids. Two (10.5%) of the 19 patients received immunosuppressive treatment. Immune-mediated colitis resolved in 89.5% of the 19 patients. Of the 10 patients in whom TEVIMBRA was withheld for colitis, 9 (90%) reinitiated TEVIMBRA after symptom improvement; of these, 2 (22%) patients had recurrence of colitis.
Immune-Mediated Hepatitis
TEVIMBRA can cause immune-mediated hepatitis, which can be fatal.
Immune-mediated hepatitis occurred in 1.3% (30/2390) of patients receiving TEVIMBRA, including Grade 4 (0.3%), Grade 3 (0.6%), and Grade 2 (0.3%) adverse reactions. Immune-mediated hepatitis led to permanent discontinuation in 6 (0.3%) patients and withholding of TEVIMBRA in 19 (0.8%) patients. Twenty-five (83.3%) of the 30 patients received systemic corticosteroids. Twenty-four (80%) of the 30 patients received high-dose systemic corticosteroids. Two (6.7%) of the 30 patients received immunosuppressive treatment. Immune-mediated hepatitis resolved in 66.7% of the 30 patients. Of the 19 patients in whom TEVIMBRA was withheld for hepatitis, 7 (37%) reinitiated TEVIMBRA after symptom improvement; of these, 1 (14%) patient had recurrence of hepatitis.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
TEVIMBRA can cause immune-mediated adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold TEVIMBRA depending on severity [see Dosage and Administration (2.2)].
Immune-mediated adrenal insufficiency occurred in 0.5% (12/2390) of patients receiving TEVIMBRA, including Grade 4 (0.04%), Grade 3 (0.2%), and Grade 2 (0.3%) adverse reactions. Adrenal insufficiency did not lead to permanent discontinuation of TEVIMBRA. TEVIMBRA was withheld in 10 (0.4%) patients. All 12 patients received systemic corticosteroids. Three (25%) of the 12 patients received high-dose systemic corticosteroids. Adrenal insufficiency resolved in 25% of the 12 patients. Of the 10 patients in whom TEVIMBRA was withheld for adrenal insufficiency, 8 (80%) reinitiated TEVIMBRA after symptom improvement; of these, none of the patients had recurrence of adrenal insufficiency.
Hypophysitis
TEVIMBRA can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as clinically indicated. Withhold or permanently discontinue TEVIMBRA depending on severity [see Dosage and Administration (2.2)].
Hypophysitis/hypopituitarism occurred in 0.3% (6/2390) of patients receiving TEVIMBRA; all were Grade 2 (0.3%). Hypophysitis did not lead to permanent discontinuation of TEVIMBRA. TEVIMBRA was withheld in 1 (0.04%) patient. Five (83.3%) of the 6 patients received systemic corticosteroids. One (17%) of the 6 patients received high-dose systemic corticosteroids. Hypophysitis/hypopituitarism resolved in 17% of the 6 patients. For the 1 patient where TEVIMBRA was withheld for hypophysitis/hypopituitarism, there was no recurrence of hypophysitis/hypopituitarism.
Thyroid Disorders
TEVIMBRA can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue TEVIMBRA depending on severity [see Dosage and Administration (2.2)].
Thyroiditis: Immune-mediated thyroiditis occurred in 1% (25/2390) of patients receiving TEVIMBRA, including Grade 2 (0.5%) adverse reactions. Thyroiditis did not lead to permanent discontinuation of TEVIMBRA. TEVIMBRA was withheld in 5 (0.2%) patients. Two (8%) of the 25 patients received systemic corticosteroids. Thyroiditis resolved in 36% of the 25 patients. All 5 patients in whom TEVIMBRA was withheld for thyroiditis reinitiated TEVIMBRA after symptom improvement; of these, 1 (20%) patient had recurrence of thyroiditis.
Hyperthyroidism: Immune-mediated hyperthyroidism occurred in 4.9% (118/2390) of patients receiving TEVIMBRA, including Grade 3 (0.04%) and Grade 2 (0.9%) adverse reactions. Hyperthyroidism led to the permanent discontinuation of TEVIMBRA in 1 (0.04%) patient and withholding of TEVIMBRA in 7 (0.3%) patients. Three (2.5%) of the 118 patients received systemic corticosteroids. Hyperthyroidism resolved in 76.3% of the 118 patients. Of the 7 patients in whom TEVIMBRA was withheld for hyperthyroidism, 5 (71.4%) reinitiated TEVIMBRA after symptom improvement; of these, none of the patients had recurrence of hyperthyroidism.
Hypothyroidism: Immune-mediated hypothyroidism occurred in 12.5% (299/2390) of patients receiving TEVIMBRA, including Grade 4 (0.04%), Grade 3 (0.04%), and Grade 2 (6.7%) adverse reactions. TEVIMBRA was permanently discontinued in 2 (0.1%) patients and treatment was withheld in 12 (0.5%) patients. Two (0.7%) of the 299 patients received systemic corticosteroids. One hundred ninety-five patients received hormone replacement therapy. Hypothyroidism resolved in 34.4% of the 299 patients. The majority (83.6%) of patients with hypothyroidism required long-term thyroid hormone replacement. Of the 12 patients in whom TEVIMBRA was withheld for hypothyroidism, 11 (91.7%) reinitiated TEVIMBRA after symptom improvement; of these, 2 (18.2%) patients had recurrence of hypothyroidism.
Type 1 Diabetes Mellitus, Which Can Present with Diabetic Ketoacidosis
Diabetes mellitus has been reported with PD-1/PD-L1 blocking antibodies. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue TEVIMBRA depending on severity [see Dosage and Administration (2.2)].
Diabetes mellitus occurred in 0.7% (16/2390) of patients receiving TEVIMBRA, including Grade 4 (0.1%), Grade 3 (0.3%), and Grade 2 (0.3%) adverse reactions. TEVIMBRA was permanently discontinued in 4 (0.2%) patients, and TEVIMBRA treatment was withheld in 4 (0.2%) patients. Fourteen of the 16 patients received insulin therapy for diabetes mellitus. Diabetes mellitus resolved in 12.5% of the 16 patients. Of the 4 patients in whom TEVIMBRA was withheld for diabetes mellitus, 1 (25%) patient reinitiated TEVIMBRA after symptom improvement.
Immune-Mediated Nephritis with Renal Dysfunction
TEVIMBRA can cause immune-mediated nephritis, which can be fatal.
Immune-mediated nephritis with renal dysfunction occurred in 0.2% (5/2390) of patients receiving TEVIMBRA, including Grade 3 (0.04%) and Grade 2 (0.1%) adverse reactions. TEVIMBRA was permanently discontinued in 1 (0.04%) patient and treatment was withheld in 3 (0.1%) patients. Three (60%) out of 5 patients received systemic corticosteroids. Three (60%) of the 5 patients received high-dose systemic corticosteroids. Nephritis with renal dysfunction resolved in 40% of the 5 patients. Of the 3 patients in whom TEVIMBRA was withheld for nephritis, 2 (66.7%) reinitiated TEVIMBRA after symptom improvement and no patients had recurrence of nephritis.
Immune-Mediated Dermatologic Adverse Reactions
TEVIMBRA can cause immune-mediated rash or dermatitis. Cases of severe cutaneous adverse reactions (SCARs), including exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), have been reported, some with fatal outcome. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue TEVIMBRA depending on severity [see Dosage and Administration (2.2)].
Immune-mediated dermatologic adverse reactions occurred in 13% (311/2390) of patients receiving TEVIMBRA, including Grade 4 (0.1%), Grade 3 (1.1%), and Grade 2 (3.4%) adverse reactions. Stevens-Johnson syndrome occurred in 1 (0.04%) patient. Dermatologic adverse reactions led to permanent discontinuation of TEVIMBRA in 3 (0.1%) patients and withholding of TEVIMBRA in 30 (1.3%) patients. Forty-four (14.1%) of the 311 patients received systemic corticosteroids. Nineteen (6.1%) of the 311 patients received high-dose systemic corticosteroids. Immune-mediated skin reactions resolved in 66.9% of the 311 patients. Of the 30 patients in whom TEVIMBRA was withheld for dermatologic adverse reactions, 26 (86.7%) reinitiated TEVIMBRA after symptom improvement; of these, 3 (12%) patients had recurrence of immune-mediated dermatologic adverse reactions.
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of less than 1% in 2390 patients who received TEVIMBRA or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions.
Cardiac/Vascular: Myocarditis, pericarditis, vasculitis.
Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barre syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis, stomatitis.
Musculoskeletal and Connective Tissue: Myositis/polymyositis/dermatomyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatica.
Endocrine: Hypoparathyroidism.
Other (Hematologic/Immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
5.2 Infusion-Related Reactions
TEVIMBRA can cause severe or life-threatening infusion-related reactions. Infusion-related reactions occurred in 4.7% (113/2390) patients receiving TEVIMBRA, including Grade 3 or higher (0.2%) reactions. Monitor patients for signs and symptoms of infusion-related reactions.
Slow the rate of infusion for mild (Grade 1) and interrupt the infusion for moderate (Grade 2) infusion-related reactions. For severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions, stop infusion and permanently discontinue TEVIMBRA [see Dosage and Administration (2.2)].
5.3 Complications of Allogeneic HSCT
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, TEVIMBRA can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TEVIMBRA and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in more detail in other sections of the label:
- Severe and fatal immune-mediated adverse reactions [see Warnings and Precautions (5.1)]
- Infusion-related reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in WARNINGS AND PRECAUTIONS reflect exposure to TEVIMBRA as a single agent in 2390 patients enrolled in three randomized open-label, active-controlled studies (BGB-A317-301, RATIONALE-302, BGB-A317-303) and six open-label, single-arm studies (BGB-A317-209, BGB-A317-208, BGB-A317-204, BGB-A317-203, BGB-A317-102, BGB-A317_Study_001), which enrolled 307 patients with esophageal squamous cell carcinoma and 2083 patients with advanced or recurrent tumors. TEVIMBRA was administered at a dose of 200 mg intravenously once every 3 weeks, except in study BGB-A317_Study_001 where patients also received other dosage regimens. Among the 2390 patients, 38% were exposed for longer than 6 months, and 23% were exposed for longer than 12 months.
First-line Treatment of Unresectable or Metastatic Esophageal Carcinoma (ESCC)
The safety of TEVIMBRA in combination with chemotherapy was evaluated in RATIONALE-306, a randomized, placebo-controlled, multicenter, double-blind trial in patients with unresectable, advanced, or metastatic ESCC [see Clinical Studies (14.1)].
Patients were randomized (1:1) to receive either TEVIMBRA 200 mg by intravenous infusion over 30-60 minutes every 3 weeks or placebo plus a chemotherapy doublet regimen. The chemotherapy doublet regimens consisted of:
- Platinum (cisplatin [60 to 80 mg/m2 IV, on Day 1] or oxaliplatin [130 mg/m2 IV, on Day 1]) and a fluoropyrimidine (5-FU [750 to 800 mg/m2 IV, on Day 1 to 5] or capecitabine [1000 mg/m2 orally twice daily, on Day 1 to 14])
or
- Platinum (cisplatin [60 to 80 mg/m2 IV, on Day 1 or 2] or oxaliplatin [130 mg/m2 IV, on Day 1 or 2]) and (paclitaxel 175 mg/m2 IV, on Day 1)
Patients were treated until disease progression or unacceptable toxicity. The median duration of exposure was 6.4 months (range: 0.1 to 38.3 months) in TEVIMBRA-treated patients.
Serious adverse reactions occurred in 48% of patients receiving TEVIMBRA in combination with chemotherapy. The most frequent serious adverse reactions (≥2%) were pneumonia (5.2%), dysphagia (5.2%), diarrhea (2.2%), fatigue (2.2%), and esophageal stenosis (2.2%). Fatal adverse reactions occurred in 8% of patients who received TEVIMBRA in combination with chemotherapy.
Permanent discontinuation of TEVIMBRA due to adverse reactions occurred in 13% of patients. The adverse reaction which resulted in discontinuation in ≥2% of patients was pneumonitis (2.2%).
Dosage interruptions of TEVIMBRA due to adverse reactions occurred in 52% of patients. Adverse reactions which required dosage interruption in ≥2% of patients were neutrophil count decreased (7%), fatigue (6%), pneumonia (6%), anemia (4.3%), neutropenia (4.3%), white blood cell count decreased (4.3%), rash (3.7%), dysphagia (2.8%), platelet count decreased (2.8%), pyrexia (2.8%), and diarrhea (2.2%).
The most common (≥20%) adverse reactions including laboratory abnormalities were decreased neutrophil count, decreased sodium, increased glucose, anemia, fatigue, decreased appetite, increased AST, decreased potassium, increased serum creatinine, decreased calcium, increased ALT, diarrhea, stomatitis, and vomiting.
Adverse reactions and laboratory abnormalities are listed in Table 3 and Table 4, respectively.
Table 3: Adverse Reactions (≥10%) in Patients with ESCC Receiving TEVIMBRA + Chemotherapy with a Difference Between Arms of ≥5% for All Grades or ≥2% for Grades 3 and 4 vs Placebo + Chemotherapy in RATIONALE-306 Adverse Reaction TEVIMBRA + Chemotherapy
N=324Placebo + Chemotherapy
N=321All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * Represents a composite of multiple, related preferred terms.
Blood and Lymphatic System Disorders Anemia 61 17 56 16 Neutropenia 16 7 15 10 General Disorders and Administration Site Conditions Fatigue* 45 9 45 4.7 Metabolism and Nutrition Disorders Decreased Appetite 44 6 39 2.2 Gastrointestinal Disorders Diarrhea 28 4.3 24 1.9 Stomatitis* 22 4 16 2.2 Vomiting 22 1.5 27 2.5 Dysphagia 14 6 11 4 Skin and Subcutaneous Tissue Disorders Rash* 19 4 9 0.3 Pruritus 13 0.3 7 0 Endocrine Disorders Hypothyroidism* 11 0 6 0 Table 4: Select Laboratory Abnormalities Worsening From Baseline Occurring in ≥10% of Patients Receiving TEVIMBRA in Combination with Chemotherapy in RATIONALE-306 with a Difference Between Arms of ≥5% for All Grades or ≥2% for Grades 3 and 4 vs Placebo + Chemotherapy in RATIONALE-306 Laboratory Abnormality TEVIMBRA + Chemotherapy*
(N=324)Placebo + Chemotherapy*
(N=321)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate varied from 132 to 323 based on the number of patients with a baseline value and at least one post-treatment value.
Hematology Neutrophils decreased 75 41 75 46 Chemistry Sodium decreased 67 19 62 11 Glucose increased 65 7 61 5 AST increased 36 3.4 27 1.3 Potassium decreased 33 10 29 2.8 Creatinine increased 33 2.5 25 1.6 Calcium decreased 29 6 24 4.4 ALT increased 28 3.1 22 1.6 Previously Treated Unresectable Advanced or Metastatic Esophageal Squamous Cell Carcinoma (ESCC)
The safety of TEVIMBRA was evaluated in RATIONALE-302, a randomized, active-controlled, open-label, multicenter study in 255 patients with unresectable advanced, recurrent or metastatic ESCC [see Clinical Studies (14.1)]. The trial excluded patients who had brain or leptomeningeal metastases that were symptomatic or required treatment, active autoimmune disease, a medical condition requiring systemic corticosteroids or immunosuppressants, or apparent tumor invasion of organs adjacent to the esophageal site.
Patients received TEVIMBRA 200 mg by intravenous infusion over 30-60 minutes every 3 weeks or investigator's choice: paclitaxel 135-175 mg/m2 every 3 weeks or 80-100 mg/m2 weekly, docetaxel 75 mg/m2 every 3 weeks, or irinotecan 125 mg/m2 on Days 1 and 8 of every 3-week cycle. Patients were treated until disease progression or unacceptable toxicity. The median duration of exposure was 2.8 months (range: 0.2 to 28.3 months) in TEVIMBRA-treated patients and 1.5 months (range: 0.2 to 19.2 months) in paclitaxel, docetaxel, or irinotecan-treated patients.
Serious adverse reactions occurred in 41% of patients; the most frequent serious adverse reactions (≥2%) were pneumonia, dysphagia, hemorrhage, pneumonitis (including pneumonitis and immune-mediated pneumonitis), and esophageal obstruction. Fatal adverse reactions occurred in 7% of patients who received TEVIMBRA, including the following which occurred in more than one patient: pneumonia/pneumonitis (5 patients), hemorrhage (3 patients), and death due to an unknown cause (3 patients).
Permanent discontinuation of TEVIMBRA due to an adverse reaction occurred in 19% of patients. Adverse reactions which resulted in permanent discontinuation in ≥1% of patients were hemorrhage, pneumonitis (including pneumonitis and immune-mediated pneumonitis), and pneumonia.
Dosage interruptions of TEVIMBRA due to an adverse reaction occurred in 23% of patients. Adverse reactions which required dosage interruptions in ≥2% of patients were pneumonia, pneumonitis, and fatigue.
The most common (≥20%) adverse reactions were anemia, fatigue, musculoskeletal pain, decreased weight, and cough.
Adverse reactions and laboratory abnormalities are listed in Table 5 and Table 6, respectively.
Table 5: Adverse Reactions (≥10%) in Patients With ESCC Receiving TEVIMBRA in RATIONALE-302 Adverse Reaction TEVIMBRA
(N=255)ICC
(N=240)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)ICC = investigator's choice of chemotherapy - * Fatigue includes asthenia, fatigue, malaise.
- † Musculoskeletal pain includes musculoskeletal pain, spinal pain, arthralgia, back pain, neck pain, musculoskeletal chest pain, myalgia, pain in extremity, non-cardiac chest pain, bone pain, arthritis.
- ‡ Cough includes productive cough, cough.
- § Pneumonia includes pneumonia aspiration, pneumonia, pneumonia bacterial, lower respiratory tract infection.
- ¶ Diarrhea includes diarrhea, colitis.
- # Abdominal pain includes abdominal pain upper, abdominal pain, abdominal discomfort, abdominal pain lower, gastrointestinal pain.
- Þ Hypothyroidism includes hypothyroidism, blood thyroid stimulating hormone increased.
- ß Rash includes dermatitis, dermatitis acneiform, dermatitis allergic, eczema, erythema, psoriasis, rash, rash follicular, rash maculo-papular, rash pruritic.
- à Hemorrhage includes tumor hemorrhage, upper gastrointestinal hemorrhage, gastrointestinal hemorrhage, hemoptysis, esophageal hemorrhage, hematuria, gastric hemorrhage, epistaxis, tracheal hemorrhage, gingival bleeding, pulmonary hemorrhage, procedural hemorrhage, rectal hemorrhage, stoma site hemorrhage.
Blood Disorders Anemia 31 6 45 11 General Disorders Fatigue* 28 2 46 6 Pyrexia 16 0.4 14 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain† 24 1 25 1 Investigations Weight decreased 23 1 19 0 Respiratory, Thoracic and Mediastinal Disorders Cough‡ 22 0.4 16 0.4 Metabolism and Nutrition Disorders Decreased appetite 16 0.4 35 4 Infections and Infestations Pneumonia§ 16 6 12 7 Gastrointestinal Disorders Constipation 15 0 19 0.4 Nausea 14 0.4 30 3 Diarrhea¶ 13 1 32 7 Dysphagia 11 6 8 3 Abdominal pain# 11 0.8 16 2 Vomiting 11 0.8 20 4 Endocrine Disorders HypothyroidismÞ 13 0.4 0.8 0 Skin and Subcutaneous Tissue Disorders Rashß 13 0.4 6 0 Vascular Disorders Hemorrhageà 12 2 10 3 Table 6: Laboratory Abnormalities Worsening From Baseline Occurring in ≥10% of Patients Receiving TEVIMBRA in RATIONALE-302 TEVIMBRA* ICC* Laboratory Abnormality All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate varied from 136 to 240 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Glucose increased 46 4 40 2 Sodium decreased 34 9 36 8 Albumin decreased 33 0.8 37 1 Alkaline phosphatase increased 32 3 15 0.5 AST increased 27 0.8 12 0.5 ALT increased 23 0.8 15 1 Phosphate decreased 15 4 20 3 Creatine kinase increased 13 1 2 0 Potassium decreased 13 1 15 3 Bilirubin increased 11 2 8 0.5 Glucose decreased 10 0.4 10 0.5 Hematology Hemoglobin decreased 45 6 61 10 Lymphocytes decreased 43 11 60 28 Platelets decreased 11 1 11 0.9 Leukocytes decreased 10 0.8 66 31 Treatment of Previously Untreated Unresectable or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma (G/GEJ)
The safety of TEVIMBRA in combination with chemotherapy was evaluated in RATIONALE-305, a randomized, multicenter, double-blind, placebo-controlled trial in patients with previously untreated unresectable or metastatic G/GEJ adenocarcinoma [see Clinical Studies (14.2)].
Patients were randomized (1:1) to receive either TEVIMBRA 200 mg by intravenous infusion over 30-60 minutes every 3 weeks or placebo plus a platinum and fluoropyrimidine-based chemotherapy. The chemotherapy regimens consisted of:
- Oxaliplatin 130 mg/m2 IV on Day 1 for up to 6 cycles and capecitabine 1000 mg/m2 orally twice daily for 14 consecutive days of every 3-week cycle
or
- Cisplatin 80 mg/m2 IV, Day 1, and 5-FU (5-fluorouracil) 800 mg/m2/day IV continuous infusion over 24 hours daily Day 1-5, every 3 weeks for up to 6 cycles
Patients were treated until disease progression or unacceptable toxicity. The median duration of exposure was 5.91 months (range: 0.1 to 47 months) in TEVIMBRA-treated patients.
Serious adverse reactions occurred in 42% of patients receiving TEVIMBRA in combination with chemotherapy. The most frequent serious adverse drug reactions (≥2%) were pneumonia (3.6%), decreased platelet count (3.2%), gastrointestinal hemorrhage (3%), and colitis (2.2%). Fatal adverse reactions occurred in 4.2% of patients who received TEVIMBRA in combination with chemotherapy; events occurring in 2 or more patients were death, sepsis, pneumonia, pulmonary embolism, and respiratory failure.
Permanent discontinuation of TEVIMBRA due to an adverse reaction occurred in 16% of patients. Adverse drug reactions which resulted in permanent discontinuation in ≥1% of patients were death, fatigue, and pneumonitis.
Dosage interruption of TEVIMBRA due to an adverse drug reaction occurred in 49% of patients. Adverse drug reactions which required dosage interruption in ≥2% of patients were decreased platelet count (12%), decreased neutrophil count (10%), neutropenia (6%), decreased white blood cell count (6%), increased AST (4.8%), increased ALT (3.8%), increased blood bilirubin (3%), COVID-19 (3%), thrombocytopenia (2.8%), leukopenia (2.6%), pneumonitis (2.2%), and pneumonia (2%).
The most common (≥20%) adverse reactions, including laboratory abnormalities, for TEVIMBRA in combination with chemotherapy were nausea, fatigue, decreased appetite, anemia, peripheral sensory neuropathy, vomiting, decreased platelet count, decreased neutrophil count, increased aspartate aminotransferase, diarrhea, abdominal pain, increased alanine aminotransferase, white blood cell count decreased, decreased weight, and pyrexia.
Adverse reactions and laboratory abnormalities are listed in Table 7 and Table 8, respectively.
Table 7: Adverse Reactions (≥10%) in Patients with G/GEJ Receiving TEVIMBRA + Chemotherapy with a Difference Between Arms of ≥5% for All Grades or ≥2% for Grades 3 and 4 vs Placebo + Chemotherapy in RATIONALE-305 Adverse Drug Reaction TEVIMBRA + Chemotherapy
(N=498)Placebo + Chemotherapy
(N=494)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * Represents a composite of multiple, related preferred terms.
General Disorders and Administration Site Conditions Pyrexia 20 1.6 14 0.6 Skin and Subcutaneous Tissue Disorders Rash* 16 1.6 7 0 Pruritus 10 0.2 3.2 0 Endocrine Disorders Hypothyroidism* 13 0.2 2.8 0 Other Clinically Important Adverse Reactions Occurring in Less Than 10% include:
Stomatitis, infusion-related reaction, dyspnea, hepatitis, hyperthyroidism, pneumonitis, hyperglycemia, myalgia, diabetes mellitus, pancreatitis, arthritis, Sjogren's syndrome, thyroiditis, adrenal insufficiency, hypophysitis, myasthenia gravis, uveitis, myocarditis, pericarditis, colitis, vitiligo, myositis, and nephritis.
Table 8: Select Laboratory Abnormalities Worsening from Baseline Occurring in ≥10% of Patients Receiving TEVIMBRA + Chemotherapy with a Difference Between Arms of ≥5% for All Grades or ≥2% for Grades 3 and 4 vs Placebo + Chemotherapy in RATIONALE-305 Laboratory Abnormality TEVIMBRA + Chemotherapy*
(N=498)Placebo + Chemotherapy*
(N=494)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)Abbreviations: ALT = alanine aminotransferase, AST = aspartate amino transferase. - * The denominator used to calculate the rate varied from 480 to 494 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry AST increased 58 6 56 3 Sodium decreased 42 7 36 5 ALT increased 41 4.8 36 2 Potassium decreased 33 9 28 6 Hematology Lymphocytes decreased 53 12 46 9 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of TEVIMBRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome, toxic epidermal necrolysis (including fatal cases).
Immune system disorders: Immune-mediated cystitis.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, TEVIMBRA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of TEVIMBRA in pregnant women. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death (see Data). Human IgG4 immunoglobulins (IgG4) are known to cross the placental barrier; therefore, tislelizumab-jsgr has the potential to be transmitted from the mother to the developing fetus. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with TEVIMBRA to evaluate its effect on reproduction and fetal development. A central function of the PD-1/PD-L1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to the fetus. In murine models of pregnancy, blockade of PD-L1 signaling has been shown to disrupt tolerance to the fetus and to result in an increase in fetal loss; therefore, potential risks of administering TEVIMBRA during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 and PD-L1 knockout mice. Based on its mechanism of action, fetal exposure to tislelizumab-jsgr may increase the risk of developing immune-mediated disorders or altering the normal immune response.
8.2 Lactation
Risk Summary
There is no information regarding the presence of tislelizumab-jsgr in human milk, or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 4 months after the last dose of TEVIMBRA.
8.3 Females and Males of Reproductive Potential
TEVIMBRA can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating TEVIMBRA [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of TEVIMBRA have not been established in pediatric patients.
8.5 Geriatric Use
TEVIMBRA as a Single Agent
Of the 255 patients who were treated with TEVIMBRA for previously treated unresectable or metastatic ESCC in the clinical study RATIONALE-302, 98 (38%) were 65 years and older and 13 (5%) were 75 years and older. No overall differences in safety or effectiveness were observed between elderly patients and younger patients.
TEVIMBRA in Combination with Chemotherapy
Of the 324 patients who were treated with TEVIMBRA and platinum-containing chemotherapy as first-line treatment for unresectable advanced or metastatic ESCC in the clinical study RATIONALE-306, 149 (46%) were 65 years and older and 13 (4%) were 75 years and older. No overall differences in safety or effectiveness were observed between elderly patients and younger patients.
Of the 498 patients who were treated with TEVIMBRA in combination with platinum-containing chemotherapy for G/GEJ adenocarcinoma in the clinical study RATIONALE-305, 161 (32%) were 65 years and older, and 28 (6%) were 75 years and older. No overall differences in safety or effectiveness were observed between elderly patients and younger patients.
-
11 DESCRIPTION
Tislelizumab-jsgr is a programmed death receptor-1 (PD-1)–blocking antibody. Tislelizumab-jsgr is an Fc-engineered humanized monoclonal IgG4 kappa antibody with an approximate molecular weight of 147 kDa. Tislelizumab-jsgr is produced in recombinant Chinese hamster ovary (CHO) cells.
TEVIMBRA (tislelizumab-jsgr) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for intravenous use, supplied in single-dose vials. Each vial contains 100 mg of tislelizumab-jsgr monoclonal antibody in 10 mL of solution, with a concentration of 10 mg/mL, and is formulated in: citric acid monohydrate (4.2 mg), histidine (17.2 mg), L-histidine hydrochloride monohydrate (8.2 mg), polysorbate 20 (2 mg), sodium citrate (59.3 mg), trehalose (650.4 mg), and Water for Injection, USP. The pH is 6.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Binding of the PD-1 ligands PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors.
Tislelizumab-jsgr binds to PD-1 and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. Tislelizumab-jsgr decreased tumor growth in xenograft models and a human PD-1 transgenic mouse model.
12.2 Pharmacodynamics
The tislelizumab-jsgr exposure-response relationship for efficacy and safety and time course of pharmacodynamic response has not been fully characterized.
12.3 Pharmacokinetics
Pharmacokinetic parameters are presented as geometric mean (% CV) unless otherwise specified.
The peak concentration (Cmax) and area under the plasma concentration versus time curve (AUC) of tislelizumab-jsgr increased dose proportionally in the dose range of 0.5 (0.2 times the approved recommended dosage in a 70 kg patient) to 10 mg/kg (3.5 times the approved recommended dosage in a 70 kg patient).
The steady-state AUCtau of tislelizumab-jsgr is 1,283 mcg/mL∙day (28.7%) and the Cmax is 110 mcg/mL (22.2%) following the approved recommended dosage. Steady-state concentration of tislelizumab-jsgr is reached after 12 weeks of repeated dosing with an every 3-week regimen and the systemic accumulation was 2.14-fold.
Elimination
The tislelizumab-jsgr total clearance is 0.153 L/day (29.5%) and the terminal half-life (t½) is 24 days (31%).
Specific Populations
No clinically significant differences in the pharmacokinetics of tislelizumab-jsgr were observed based on age (range: 18 to 90 years), weight (range: 32 to 130 kg), race (White, Asian, or Black), mild to moderate renal impairment (CLcr ≥30 mL/min, estimated by Cockcroft-Gault), mild to moderate hepatic impairment (total bilirubin ≤3 times ULN and any AST, estimated by NCI criteria). The effect of severe hepatic impairment (total bilirubin >3 times ULN and any AST), severe renal impairment (CLcr 15-29 mL/min), or end-stage renal disease (CLcr <15 mL/min) on the pharmacokinetics of tislelizumab-jsgr is unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of tislelizumab-jsgr products.
In patients who received tislelizumab-jsgr in RATIONALE-306 for up to 26 months, the incidence of anti-tislelizumab antibodies was 22% (66/300). Among the anti-tislelizumab antibody-positive patients, the incidence of neutralizing antibodies was 1.5% (1/66).
In patients who received tislelizumab-jsgr in RATIONALE-302 for up to 22 months, the incidence of anti-tislelizumab antibodies was 14.5% (32/221). Among the anti-tislelizumab antibody-positive patients, the incidence of neutralizing antibodies was 3.1% (1/32).
In patients who received tislelizumab-jsgr in RATIONALE-305 throughout the treatment period and in the ADA analysis set, the incidence of anti-tislelizumab antibodies was 22.7% (108/475). Among the anti-tislelizumab antibody-positive patients, the incidence of neutralizing antibodies was 5.6% (6/108).
There was no significant effect of anti-drug antibodies on the pharmacokinetics of tislelizumab-jsgr. The effect of anti-drug antibodies on pharmacodynamics, safety, or effectiveness of tislelizumab-jsgr has not been fully characterized.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the potential of tislelizumab-jsgr for carcinogenicity or genotoxicity.
In a 3-month repeat-dose toxicology study in cynomolgus monkeys, there were no notable effects in the male and female reproductive organs; however, most animals in the study were not sexually mature.
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-L1/PD-1 signaling resulted in an increased severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis–infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti–PD-1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-L1 and PD-1 knockout mice have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
-
14 CLINICAL STUDIES
14.1 Esophageal Squamous Cell Carcinoma
First-line Treatment of Unresectable or Metastatic Esophageal Carcinoma (ESCC) in Patients Whose Tumors Express PD-L1 (≥1)
The efficacy of TEVIMBRA, in combination with chemotherapy, was evaluated in RATIONALE-306 (NCT03783442), a global, randomized, placebo-controlled, double-blind study in patients with unresectable, recurrent, or metastatic esophageal squamous cell carcinoma (ESCC).
Patients were enrolled regardless of their PD-L1 expression level. PD-L1 expression was evaluated at a central laboratory using the Ventana PD-L1 (SP263) assay that identified PD-L1 staining on both tumor and tumor-associated immune cells (Tumor Area Positivity or TAP). A retrospective scoring of tumor PD-L1 status using Combined Positive Score (CPS) was also conducted using the PD-L1–stained tumor specimens used for randomization.
Patients should not have received prior systemic therapy for advanced or metastatic disease. A treatment-free interval of at least 6 months was required if there was prior neoadjuvant/adjuvant therapy with platinum-based chemotherapy. The trial excluded patients who had active leptomeningeal disease or uncontrolled brain metastasis, active autoimmune disease, a medical condition requiring systemic corticosteroids or immunosuppressants, or evidence of fistula or complete esophageal obstruction not amenable to treatment.
Patients were randomized (1:1) to receive either TEVIMBRA 200 mg every 3 weeks or placebo in combination with investigator's choice of chemotherapy (ICC) on a 21-day cycle. Patients received TEVIMBRA until disease progression assessed by the investigator per RECIST v1.1, or until unacceptable toxicity. The chemotherapy doublet regimen consists of:
- Platinum (cisplatin [60 to 80 mg/m2 IV, on Day 1] or oxaliplatin [130 mg/m2 IV, on Day 1]) and a fluoropyrimidine (fluorouracil [750 to 800 mg/m2 IV, on Days 1 to 5] or capecitabine [1000 mg/m2 orally twice daily, on Days 1 to 14])
or
- Platinum (cisplatin [60 to 80 mg/m2 IV, on Day 1 or 2] or oxaliplatin [130 mg/m2 IV, on Day 1 or 2]) and (paclitaxel 175 mg/m2 IV, on Day 1)
Cross-over between treatment arms or between fluoropyrimidine and paclitaxel during the study treatment period was not allowed.
Patient randomization was stratified by geographic region (Asia [excluding Japan] versus Japan versus Rest of World), prior definitive therapy (yes versus no), and investigator choice of chemotherapy (ICC; platinum with fluoropyrimidine versus platinum with paclitaxel).
Tumor assessments were performed every 6 weeks for the first 48 weeks, then every 9 weeks thereafter.
The primary efficacy outcome measure was overall survival (OS) in the Intent-to-Treat (ITT) population. Secondary outcome measures included progression-free survival (PFS), objective response rate (ORR), and duration of response (DoR) as assessed by the investigator per RECIST v1.1. Additional analyses of efficacy outcome measures were also conducted based on PD-L1 TAP ≥1% and CPS ≥1.
A total of 649 patients were randomized. The trial population characteristics were median age 64 years (range: 26 to 84 years), 48% were ≥65 years of age, 87% were male, 75% were Asian, and 24% were White. Eighty-six percent had metastatic disease and 14% had locally advanced disease; 99.8% of patients had histological confirmation of squamous cell carcinoma. Baseline ECOG performance status was 0 (33%) or 1 (67%). Thirty-four percent of patients had tumors that expressed PD-L1 TAP ≥10%, 74% had PD-L1 TAP ≥1%, and 74% had PD-L1 CPS ≥1. Fifty-five percent of patients received platinum (cisplatin or oxaliplatin) and paclitaxel-containing regimens, and 45% received platinum (cisplatin or oxaliplatin) and fluoropyrimidine-containing regimens.
RATIONALE-306 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA in combination with chemotherapy compared to placebo in combination with chemotherapy. Exploratory analysis of OS in the population with TAP <1% population and in the CPS <1 population showed hazard ratios of 1.34 (95% CI 0.73, 2.46) and 1.52 (95% CI 0.81, 2.84), respectively, indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 ≥1.
Efficacy results are shown in Table 9, Figure 1, and Figure 2.
Table 9: Efficacy results in RATIONALE-306 Endpoint TEVIMBRA + Chemotherapy
(N=231)Placebo + Chemotherapy
(N=250)TEVIMBRA + Chemotherapy
(N=233)Placebo + Chemotherapy
(N=247)PD-L1 TAP ≥1% PD-L1 CPS ≥1 CI = confidence interval; HR = hazard ratio. - * Medians were estimated by Kaplan-Meier method with 95% CIs estimated using the method of Brookmeyer and Crowley.
- † Estimated by Cox proportional hazards model.
- ‡ Confirmed responses.
- § Exact Clopper-Person-2-sided CI.
Overall Survival (OS) Deaths n (%) 141 (61) 177 (70.8) 141 (61) 175 (71) Median (months)* (95% CI) 16.8 (15.3, 20.8) 9.6 (8.9, 11.8) 16.8 (15.3, 20.8) 9.6 (8.9, 11.8) HR† (95% CI) 0.66 (0.53, 0.82) 0.65 (0.52,0.81) Progression-Free Survival (PFS) Events, n (%) 152 (66) 199 (80) 153 (66) 195 (79) Median (months)* (95% CI) 7.2 (6.8, 8.5) 5.5 (4.5, 5.8) 7.1 (6.8, 8.3) 5.5 (4.5, 5.8) HR† (95% CI) 0.56 (0.45, 0.70) 0.57 (0.46, 0.71) Objective Response Rate (ORR)‡ Responders, n 134 90 134 89 ORR, % 58 36 58 36 95% CI§ (51, 65) (30, 42) (51, 64) (30, 42) Complete response (CR), n (%) 11 (4.8) 5 (2) 11 (4.7) 5 (2) Partial response, n (%) 123 (53) 85 (34) 123 (53) 84 (34) Duration of Response (DoR) Median DoR (months)* (95% CI) 7.2 (6.2, 9.6) 5.7 (4.4, 7.3) 7.6 (6.6, 9.7) 5.6 (4.4, 7.3) Figure 1: Kaplan-Meier Curve for Overall Survival in RATIONALE-306 (PD-L1 TAP ≥1%)
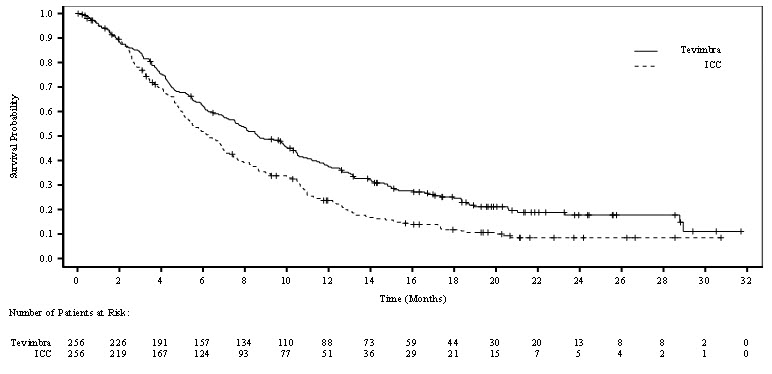
Figure 2: Kaplan-Meier Curve for Overall Survival in RATIONALE-306 (PD-L1 CPS ≥1)
Efficacy results from the exploratory retrospective analysis with CPS scoring were generally consistent with the efficacy results for TAP subgroups detailed in Table 9 and Figure 1.
Previously Treated Unresectable or Metastatic Esophageal Squamous Cell Carcinoma (ESCC)
RATIONALE-302 (NCT03430843) was a multicenter, randomized (1:1), open-label trial in 512 adult patients with unresectable advanced or metastatic ESCC who progressed on or after prior systemic chemotherapy.
Patients were enrolled regardless of their tumor PD-L1 expression level. PD-L1 expression was evaluated at a central laboratory using the Ventana PD-L1 (SP263) assay that identified PD-L1 staining on both tumor and tumor-associated immune cells (TAP). The trial excluded patients who received a prior immune checkpoint inhibitor, had brain or leptomeningeal metastases that were symptomatic or required treatment, active autoimmune disease, a medical condition requiring systemic corticosteroids or immunosuppressants, or apparent tumor invasion of organs adjacent to the esophageal tumor.
Patients were randomized (1:1) to receive either TEVIMBRA 200 mg every 3 weeks or investigator's choice of chemotherapy (ICC), all given intravenously: paclitaxel 135-175 mg/m2 every 3 weeks or 80 to 100 mg/m2 weekly, docetaxel 75 mg/m2 every 3 weeks, or irinotecan 125 mg/m2 on Days 1 and 8 of every 3-week cycle. Patients were treated until disease progression assessed by the investigator or unacceptable toxicity.
Randomization was stratified by geographic region (Asia [excluding Japan] vs Japan vs US/EU), ECOG performance status (0 vs 1), and ICC option. Tumor assessments were conducted every 6 weeks for the first 6 months, then every 9 weeks until disease progression.
The major efficacy outcome measure was overall survival (OS) in the Intent-to-Treat (ITT) population. Additional efficacy outcome measures were investigator-assessed progression-free survival (PFS), overall response rate (ORR), and duration of response (DOR) per RECIST v1.1.
A total of 512 patients were enrolled and randomized to TEVIMBRA (n=256) or ICC (n=256) (irinotecan [46%], paclitaxel [33%], or docetaxel [21%]). Of the 512 patients, 142 (28%) had PD-L1 ≥10%, 222 (43%) had PD-L1 <10%, and 148 (29%) had unknown baseline PD-L1 status.
The trial population characteristics were: median age of 62 years (range: 35 to 86), 38% age ≥65; 84% male; 19% White and 80% Asian; 95% had metastatic disease. All patients had received at least one prior anti-cancer systemic therapy. Baseline ECOG performance status was 0 (25%) or 1 (75%).
RATIONALE-302 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA as compared with ICC. OS results by PD-L1 CPS level (<1 and ≥1) were not studied.
Efficacy results are shown in Table 10 and Figure 3.
Table 10: Efficacy Results in RATIONALE-302 in ITT Population Endpoint TEVIMBRA
(N=256)ICC
(N=256)CI = confidence interval, ORR = objective response rate. - * Estimated using Kaplan-Meier method.
- † Based on Cox regression model stratified by baseline ECOG status and ICC option.
- ‡ One-sided p-value based on log-rank test stratified by ECOG performance status and ICC option.
- § Confirmed response.
Overall Survival Deaths n (%) 197 (77) 213 (83.2) Median (months)* (95% CI) 8.6 (7.5, 10.4) 6.3 (5.3, 7) Hazard ratio† (95% CI) 0.7 (0.57, 0.85) p-value‡ 0.0001 Progression-Free Survival Disease progression or death (%) 223 (87.1) 180 (70.3) Median (months)* (95% CI) 1.6 (1.4, 2.7) 2.1 (1.5, 2.7) Hazard ratio† (95% CI) 0.83 (0.67, 1.01) Objective Response Rate§ ORR (%) (95% CI) 15.2 (11.1, 20.2) 6.6 (3.9, 10.4) Complete response n (%) 5 (2) 1 (0.4) Partial response n (%) 34 (13.3) 16 (6.3) Duration of Response Median (months)* (95% CI) 10.3 (6.5, 13.2) 6.3 (2.8, 8.5) Figure 3: Kaplan-Meier Curve for Overall Survival in RATIONALE-302 (ITT)
14.2 Gastric Cancer
Previously Untreated, Unresectable, or Metastatic HER2-Negative Gastric or Gastroesophageal Junction (G/GEJ) Adenocarcinoma in Patients Whose Tumors Express PD-L1 (≥1)
RATIONALE-305 (NCT03777657) was a randomized, multicenter, placebo-controlled, double-blind trial in patients with HER2-negative previously untreated unresectable or metastatic G/GEJ adenocarcinoma.
Patients were enrolled regardless of their tumor PD-L1 expression level, which was evaluated prospectively at a central laboratory using the VENTANA PD-L1 (SP263) assay that identified PD-L1 staining on both tumor and tumor-associated immune cells (TAP). A retrospective scoring of tumor PD-L1 status using Combined Positive Score (CPS) was also conducted using the PD-L1-stained tumor specimens used for randomization.
The trial excluded patients who had active leptomeningeal disease or uncontrolled brain metastasis, and patients with active autoimmune disease or history of autoimmune diseases, or a medical condition requiring systemic corticosteroids or immunosuppressants.
Patients were randomized to receive either TEVIMBRA 200 mg every 3 weeks or placebo in combination with investigator's choice of chemotherapy on a 21-day cycle. TEVIMBRA (or placebo) was administered until disease progression or unacceptable toxicity.
The chemotherapy doublets regimen consisted of:
- CAPOX: Oxaliplatin 130 mg/m2 IV on Day 1 for up to 6 cycles and capecitabine 1000 mg/m2 orally twice daily for 14 consecutive days. Capecitabine treatment could be continued beyond 6 cycles
or
- FP: Cisplatin 80 mg/m2 IV, Day 1, and 5-FU 800 mg/m2/day IV continuous infusion over 24 hours daily Day 1-5. Cisplatin and 5-FU were given for up to 6 cycles
Cross-over between treatment arms was not allowed.
Patient randomization was stratified by geographic region (China [including Taiwan], vs Japan and South Korea vs rest of the world, including US and Europe); PD-L1 expression (PD-L1 TAP score ≥5% vs PD-L1 TAP score <5%); presence of peritoneal metastasis (yes vs no); and ICC option (oxaliplatin plus capecitabine vs cisplatin plus 5-FU).
Tumor assessments were performed every 6 weeks for the first 48 weeks and thereafter approximately every 9 weeks.
The primary efficacy outcome measures were OS in the PD-L1 TAP score ≥5% population and in the Intent-to-Treat (ITT) population. Secondary outcome measures included progression-free survival (PFS), objective response rate (ORR), and duration of response (DoR) as assessed by the investigator per RECIST v1.1. Additional analyses of efficacy outcome measures were also conducted based on PD-L1 TAP ≥1% and CPS ≥1.
A total of 997 patients were randomized. The trial population characteristics were median age 61 years (range: 23 to 86 years), 35% ≥65 years of age, 69% male; 75% Asian, 22% White, and 0% Black or African American. Eighty percent had primary stomach tumor; 89% had PD-L1 TAP ≥1% and 86% had PD-L1 CPS ≥1, and 99% of patients had metastatic disease at baseline. Baseline ECOG performance status was 0 (32%) or 1 (68%). Ninety-three percent of patients received CAPOX and 7% received FP.
RATIONALE-305 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA in combination with chemotherapy compared with placebo plus chemotherapy in the PD-L1 TAP ≥5% population and in the ITT population. Exploratory analyses of OS in the TAP <1% population and in the CPS <1 population showed hazard ratios of 0.98 (95% CI: 0.64, 1.50) and 1.01 (95% CI: 0.66, 1.52) respectively, indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 ≥1.
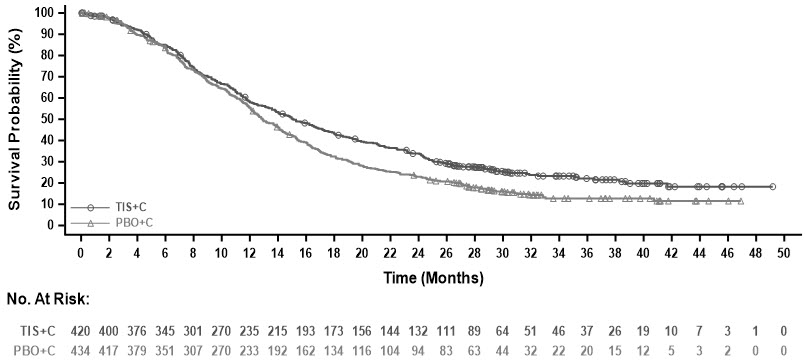
Efficacy results are summarized in Table 11, Figure 4, and Figure 5.
Table 11: Efficacy Results in RATIONALE-305 Endpoint TEVIMBRA + Chemotherapy
(N=432)Placebo + Chemotherapy
(N=453)TEVIMBRA + Chemotherapy
(N=420)Placebo + Chemotherapy
(N=434)PD-L1 TAP ≥1% PD-L1 CPS ≥1 Abbreviations: CI = confidence interval, HR = hazard ratio, ORR = objective response rate. - * Medians were estimated by Kaplan-Meier method with 95% CIs estimated using the method of Brookmeyer and Crowley.
- † Estimated by Cox proportional hazards model.
- ‡ Based on confirmed response.
- § Exact Clopper-Pearson 2-sided confidence interval.
Overall Survival Deaths n (%) 318 (74) 370 (82) 308 (73) 356 (82) Median (months)* (95% CI) 15.0 (13.3, 16.7) 12.8 (12.1, 14.1) 15.1 (13.6, 17.2) 12.9 (12.1, 14.1) HR† (95% CI) 0.78 (0.67, 0.90) 0.78 (0.67, 0.91) Progression-Free Survival Events, n (%) 316 (73) 364 (80) 303 (72) 348 (80) Median‡ (months) (95% CI) 6.9 (5.7, 7.2) 5.9 (5.6, 6.9) 7.0 (5.7, 7.7) 6.4 (5.6, 6.9) HR† (95% CI) 0.78 (0.67, 0.91) 0.77 (0.66, 0.90) Objective Response Rate‡ ORR, n 206 186 204 183 ORR, % 48 41 49 42 95% CI (%)§ (43, 53) (37, 46) (44, 53) (37, 47) Complete response, n (%) 15 (3.5) 15 (3.3) 16 (3.8) 16 (3.7) Partial response, n (%) 191 (44) 171 (38) 188 (45) 167 (38) Duration of Response Median (months)* (95% CI) 8.6 (7.8, 10.4) 7.2 (5.8, 8.3) 8.6 (7.8, 10.4) 7.2 (5.8, 8.5) Figure 4: Kaplan-Meier Curve for Overall Survival in RATIONALE-305 (PD-L1 TAP ≥1%)
Figure 5: Kaplan-Meier Curve for Overall Survival in RATIONALE-305 (PD-L1 CPS ≥1)
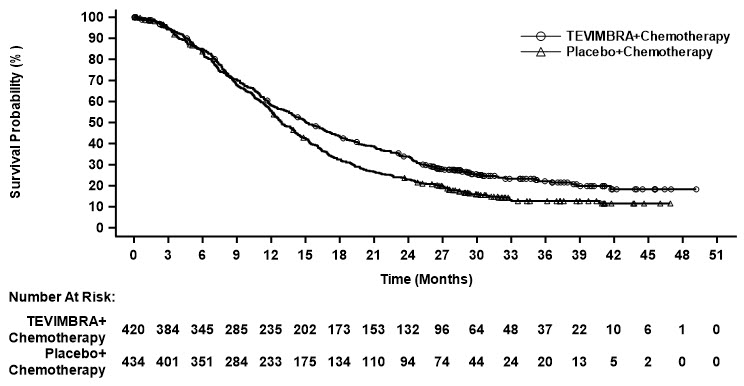
An exploratory subgroup analysis of OS in 40 patients with MSI-H tumors irrespective of PD-L1 status showed a HR of 0.66 (0.3, 1.43).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
TEVIMBRA injection is a clear to slightly opalescent, colorless to slightly yellow solution supplied in a carton containing one 100 mg/10 mL (10 mg/mL) single-dose vial (NDC: 72579-121-01).
-
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of TEVIMBRA. These reactions may include:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for new or worsening cough, chest pain, or shortness of breath [see Warnings and Precautions (5.1)].
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea, or severe abdominal pain [see Warnings and Precautions (5.1)].
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, pain on the right side of the abdomen, or easy bruising or bleeding [see Warnings and Precautions (5.1)].
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of hypophysitis, adrenal insufficiency, hypothyroidism, hyperthyroidism, thyroiditis, or Type 1 diabetes mellitus [see Warnings and Precautions (5.1)].
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis [see Warnings and Precautions (5.1)].
- Dermatologic Adverse Reactions: Advise patients to contact their healthcare provider immediately for any signs or symptoms of severe skin reactions, SJS, TEN, or DRESS [see Warnings and Precautions (5.1)].
- Other Immune-Mediated Adverse Reactions:
- Advise patients that immune-mediated adverse reactions can occur and may involve any organ system, and to contact their healthcare provider immediately for any new or worsening signs or symptoms [see Warnings and Precautions (5.1)].
- Advise patients of the risk of solid organ transplant rejection and other transplant (including corneal graft) rejection and to contact their healthcare provider immediately for signs or symptoms of organ transplant rejection [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.2)].
Complications of Allogeneic Hematopoietic Stem Cell Transplantation Complications
Advise patients of potential risk of post-allogeneic hematopoietic stem cell transplantation complications (HSCT) [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
Advise females of reproductive potential to use effective contraception during treatment with TEVIMBRA and for 4 months after the last dose [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with TEVIMBRA and for 4 months after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Medication Guide
TEVIMBRA® (teh-vim-brah)
(tislelizumab-jsgr)
injectionThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 12/2025 What is the most important information I should know about TEVIMBRA?
TEVIMBRA is a medicine that may treat certain cancers by working with your immune system. TEVIMBRA can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended.
Call or see your healthcare provider right away if you develop any new or worsening symptoms, including:
Lung problems- new or worsening cough
- shortness of breath
- chest pain
Intestinal problems - diarrhea (loose stools) or more bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
- yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems - headaches that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems - decrease in your amount of urine
- blood in your urine
- swelling in your ankles
- loss of appetite
Skin problems - rash
- itching
- skin blistering or peeling
- painful sores or ulcers in your mouth or in your nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with TEVIMBRA. Call or see your healthcare provider right away for new or worsening symptoms, which may include: - chest pain, irregular heartbeat, shortness of breath, swelling of ankles
- confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- double vision, blurry vision, sensitivity to light, eye pain, changes in eyesight
- persistent or severe muscle pain or weakness, muscle cramps
- low red blood cells, bruising
- chills or shaking
- itching or rash
- flushing
- shortness of breath or wheezing
- dizziness
- feeling like passing out
- fever
- back or neck pain
Rejection of a transplanted organ or tissue. Your healthcare provider should tell you what signs and symptoms you should report and monitor you, depending on the type of organ or tissue transplant that you have had.
Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with TEVIMBRA. Your healthcare provider will monitor you for these complications.
Getting medical treatment right away may help keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during your treatment with TEVIMBRA. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with TEVIMBRA if you have severe side effects.What is TEVIMBRA?
TEVIMBRA is a prescription medicine used to treat adults with:- cancer of the tube that connects your throat to your stomach (esophageal cancer).
- TEVIMBRA may be used in combination with chemotherapy that contains platinum as your first treatment when your esophageal cancer:
- is a type called squamous cell carcinoma, and
- cannot be removed with surgery or has spread to other parts of the body (metastatic), and
- your tumor tests positive for "PD-L1."
- TEVIMBRA may be used alone when your esophageal cancer:
- is a type called squamous cell carcinoma, and
- cannot be removed with surgery or has spread to other parts of the body (metastatic), and
- you have had previous treatment that did not include a PD-(L)1 inhibitor medicine.
- TEVIMBRA may be used in combination with chemotherapy that contains platinum as your first treatment when your esophageal cancer:
- cancer of the stomach (gastric cancer) or cancer where the esophagus joins the stomach (gastroesophageal junction cancer).
- TEVIMBRA may be used in combination with chemotherapy that contains platinum and fluoropyrimidine as your first treatment when your gastric or gastroesophageal junction cancer:
- cannot be removed with surgery or has spread to other parts of the body (metastatic), and
- your tumor tests positive for "PD-L1."
- TEVIMBRA may be used in combination with chemotherapy that contains platinum and fluoropyrimidine as your first treatment when your gastric or gastroesophageal junction cancer:
Before receiving TEVIMBRA, tell your healthcare provider about all of your medical conditions, including if you: - have immune system problems such as Crohn's disease, ulcerative colitis, or lupus
- have received an organ or tissue transplant, including corneal transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have received radiation treatment to your chest area
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- are pregnant or plan to become pregnant. TEVIMBRA can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider will do a pregnancy test before you start treatment with TEVIMBRA.
- You should use an effective method of birth control during your treatment and for 4 months after your last dose of TEVIMBRA. Talk to your healthcare provider about birth control methods that you can use during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with TEVIMBRA.
- are breastfeeding or plan to breastfeed. It is not known if TEVIMBRA passes into your breast milk. Do not breastfeed during treatment with TEVIMBRA and for 4 months after your last dose of TEVIMBRA.
How will I receive TEVIMBRA? - Your healthcare provider will give you TEVIMBRA into your vein through an intravenous (IV) line over 30 to 120 minutes depending on the dose you are receiving.
- TEVIMBRA is usually given every 2, 3, 4, or 6 weeks depending on the dose you are receiving.
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider will do blood tests to check you for certain side effects.
- If you miss any appointment, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of TEVIMBRA?
TEVIMBRA may cause serious side effects. See "What is the most important information I should know about TEVIMBRA?"
The most common side effects of TEVIMBRA when used in combination with platinum-containing chemotherapy include:- decreased white blood cell count
- decreased salt (sodium) in your blood
- increased glucose in your blood
- decreased red blood cell count (anemia)
- tiredness
- decreased appetite
- increase in certain liver blood tests
- decreased potassium in your blood
- increase in certain kidney blood tests
- decreased calcium in your blood
- diarrhea
- mouth sores
- vomiting
The most common side effects of TEVIMBRA when used alone include: - increased blood sugar
- decreased red blood cell (anemia) and white blood cell counts
- decreased salt (sodium) in your blood
- decreased albumin in the blood
- increase in certain liver blood tests
- tiredness
- muscle and bone pain
- decreased weight
- cough
The most common side effects of TEVIMBRA in combination with platinum and fluoropyrimidine-based chemotherapy include: - nausea
- tiredness
- decreased appetite
- decreased red blood cell count (anemia)
- numbness, pain, tingling, or burning in your hands or feet
- vomiting
- decreased platelet count
- decreased white blood cell count
- increase in certain liver blood tests
- diarrhea
- stomach-area (abdominal) pain
- decreased weight
- fever
These are not all the possible side effects of TEVIMBRA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of TEVIMBRA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider or pharmacist for more information about TEVIMBRA that is written for health professionals.What are the ingredients in TEVIMBRA?
Active ingredient: tislelizumab-jsgr
Inactive ingredients: citric acid monohydrate, histidine, L-histidine hydrochloride monohydrate, polysorbate 20, sodium citrate, trehalose, and Water for Injection.
Manufactured by: BeOne Medicines USA, Inc. Pennington, NJ 08534
U.S. License No. 2400
TEVIMBRA® is a registered trademark owned by BeOne Medicines I GmbH or its affiliates.
© BeOne Medicines I GmbH, 2025. All Rights Reserved.
For more information, call 1-833-969-2463 or go to www.TEVIMBRA.com -
PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
NDC: 72579-121-01
Rx onlyTEVIMBRA®
tislelizumab-jsgr
injection100 mg/10 mL
(10 mg/mL)For Intravenous Infusion
After DilutionDispense with Medication Guide
One 10 mL single-dose vial
Discard unused portion
-
INGREDIENTS AND APPEARANCE
TEVIMBRA
tislelizumab-jsgr injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72579-121 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TISLELIZUMAB (UNII: 0KVO411B3N) (TISLELIZUMAB - UNII:0KVO411B3N) TISLELIZUMAB 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.42 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 2.54 mg in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.2 mg in 1 mL SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) 5.93 mg in 1 mL TREHALOSE (UNII: B8WCK70T7I) 65.04 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72579-121-01 1 in 1 CARTON 03/14/2024 1 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761232 03/14/2024 Labeler - BeOne Medicines USA, Inc. (119367185) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Biopharmaceuticals (China) Ltd. 544325726 ANALYSIS(72579-121) , MANUFACTURE(72579-121) , API MANUFACTURE(72579-121) Establishment Name Address ID/FEI Business Operations BeOne Pharmaceutical (Suzhou) Co.,Ltd 543941083 ANALYSIS(72579-121) Establishment Name Address ID/FEI Business Operations Sharp Packaging Services, LLC 117503828 PACK(72579-121) , LABEL(72579-121) Establishment Name Address ID/FEI Business Operations Microcoat Biotechnologie GmbH 343903364 ANALYSIS(72579-121) Establishment Name Address ID/FEI Business Operations Lek d.d. 364704288 ANALYSIS(72579-121) Establishment Name Address ID/FEI Business Operations BioReliance Ltd. 505004556 ANALYSIS(72579-121)
Trademark Results [TEVIMBRA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TEVIMBRA 79314253 not registered Live/Pending |
NOVARTIS AG 2021-04-30 |
 TEVIMBRA 79228266 5537974 Live/Registered |
NOVARTIS AG 2018-01-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.