DOPAMINE HYDROCHLORIDE IN DEXTROSE injection, solution
Dopamine Hydrochloride in Dextrose by
Drug Labeling and Warnings
Dopamine Hydrochloride in Dextrose by is a Prescription medication manufactured, distributed, or labeled by Hospira, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Dopamine Hydrochloride in 5% Dextrose Injection, USP is a sterile, nonpyrogenic, prediluted solution of dopamine hydrochloride in 5% dextrose injection. It is administered by intravenous infusion.
Each 100 mL contains dopamine hydrochloride 80 mg (0.8 mg/mL), 160 mg (1.6 mg/mL) or 320 mg (3.2 mg/mL) and dextrose, hydrous 5 g in water for injection, with sodium metabisulfite added 50 mg as a stabilizer; osmolar concentration, respectively 261, 269, or 286 mOsmol/liter (calc.), pH 3.8 (2.5 to 4.5). May contain hydrochloric acid and/or sodium hydroxide for pH adjustment.
Dopamine administered intravenously is a myocardial inotropic agent, which also may increase mesenteric and renal blood flow plus urinary output.
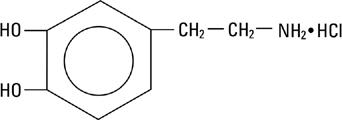
Dopamine Hydrochloride is chemically designated 3, 4-dihydroxyphenethylamine hydrochloride (C8H11NO2 HCl), a white crystalline powder freely soluble in water. It has the following structural formula:
Dopamine (also referred to as 3-hydroxytyramine) is a naturally occurring endogenous catecholamine precursor of norepinephrine.
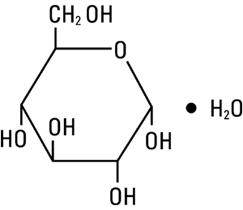
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated CR3 plastic material. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
-
CLINICAL PHARMACOLOGY
Dopamine exhibits an inotropic action on the myocardium, resulting in increased cardiac output. It causes less increase in myocardial oxygen consumption than isoproterenol and the effect of dopamine usually is not associated with tachyarrhythmia. Reported clinical studies have revealed that the drug usually increases systolic and pulse pressure without any or only a minor elevating effect on diastolic pressure. Total peripheral resistance at low and intermediate doses is usually unchanged. Blood flow to peripheral vascular beds may decrease while mesenteric blood flow is increased. The drug also has been reported to produce dilation of the renal vasculature which is accompanied by increases in glomerular filtration rate, renal blood flow and sodium excretion. Increased urinary output produced by dopamine is usually not associated with decreased urine osmolality.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss due to perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
The reported clearance rate of dopamine in critically ill infants and children has ranged from 46 to 168 mL/kg/min, with the higher values seen in the younger patients. The apparent volume of distribution in neonates is reported as 0.6 to 4 L/kg, leading to an elimination half-life of 5 to 11 minutes.
-
INDICATIONS AND USAGE
Dopamine Hydrochloride in 5% Dextrose Injection, USP is indicated for the correction of hemodynamic imbalances present in shock due to myocardial infarction, trauma, endotoxic septicemia, open heart surgery, renal failure and chronic cardiac decompensation as in refractory congestive failure.
When indicated, restoration of circulatory volume should be instituted or completed with a suitable plasma expander or whole blood, prior to administration of dopamine hydrochloride.
Patients most likely to respond to dopamine are those whose physiological parameters (such as urine flow, myocardial function and blood pressure) have not undergone extreme deterioration. Reports indicate that the shorter the time between onset of signs and symptoms and initiation of therapy with volume restoration and dopamine, the better the prognosis.
Poor Perfusion of Vital Organs: Although urine flow is apparently one of the better diagnostic signs for monitoring vital organ perfusion, the physician also should observe the patient for signs of reversal of mental confusion or coma. Loss of pallor, increase in toe temperature or adequacy of nail bed capillary filling also may be observed as indices of adequate dosage. Reported studies indicate that when dopamine is administered before urine flow has decreased to approximately 0.3 mL/minute prognosis is more favorable.
However, it has been observed that in some oliguric or anuric patients, administration of the drug has produced an increase in urine flow which may reach normal levels. The drug also may increase urine flow in patients whose output is within normal limits and thus may help in reducing the degree of pre-existing fluid accumulation. Conversely, at higher than optimal doses for a given patient, urinary flow may decrease, requiring a reduction of dosage. Concomitant administration of dopamine and diuretic agents may produce an additive or potentiating effect.
Low Cardiac Output: Dopamine's direct inotropic effect on the myocardium which increases cardiac output at low or moderate doses is related to a favorable prognosis. Increased output has been associated with unchanged or decreased systemic vascular resistance (SVR). The association of static or decreased SVR with low or moderate increases in cardiac output is regarded as a reflection of differential effects on specific vascular beds, with increased resistance in peripheral beds (e.g., femoral), and concurrent decreases in mesenteric and renal vascular beds. Redistribution of blood flow parallels these changes so that an increase in cardiac output is accompanied by an increase in mesenteric and renal blood flow. In many instances the renal fraction of the total cardiac output has been found to increase. Increase in cardiac output produced by dopamine is not associated with substantial decreases in systemic vascular resistance as may occur with isoproterenol.
Hypotension: Low to moderate doses of dopamine, which have little effect on SVR, can be used to manage hypotension due to inadequate cardiac output. At high therapeutic doses, dopamine's α-adrenergic action becomes more prominent and thus may correct hypotension due to diminished SVR. As in other circulatory decompensation states, prognosis is better in patients whose blood pressure and urine flow have not undergone extreme deterioration. Therefore, it is suggested the physician administer dopamine as soon as a definite trend toward decreased systolic and diastolic pressure becomes apparent.
- CONTRAINDICATIONS
-
WARNINGS
Do NOT add any alkalinizing substance, since dopamine is inactivated in alkaline solution.
Patients who have been treated with monoamine oxidase (MAO) inhibitors prior to administration of dopamine should receive substantially reduced dosage of the latter. See PRECAUTIONS, Drug Interactions, below.
Additive medications should not be delivered via this solution.
Dopamine Hydrochloride in 5% Dextrose Injection, USP contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
-
PRECAUTIONS
General:
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Fluid and Electrolyte Balance: Excess administration of potassium-free solutions may result in significant hypokalemia.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
Careful Monitoring Required: Close monitoring of the following indices ─ urine flow, cardiac output and blood pressure ─ during dopamine infusion is necessary as in the case of any adrenergic agent.
Hypoxia, Hypercapnia, Acidosis: These conditions, which may also reduce the effectiveness and/or increase the incidence of adverse effects of dopamine, must be identified and corrected prior to, or concurrently with, administration of dopamine HCl.
Ventricular Arrhythmias: If an increased number of ectopic beats are observed the dose should be reduced if possible.
Hypotension: At lower infusion rates, if hypotension occurs, the infusion rate should be rapidly increased until adequate blood pressure is obtained. If hypotension persists, dopamine HCl should be discontinued and a more potent vasoconstrictor agent such as norepinephrine should be administered.
Occlusive Vascular Disease: Patients with a history of occlusive vascular disease (e.g., arteriosclerosis, arterial embolism, Raynaud's disease, cold injury such as frostbite, diabetic endarteritis, and Buerger's disease) should be closely monitored for any changes in color or temperature of the skin of the extremities. If a change in skin color or temperature occurs and is thought to be the result of compromised circulation to the extremities, the benefits of continued dopamine infusion should be weighed against the risk of possible necrosis. These changes may be reversed by decreasing the rate or discontinuing the infusion entirely.
Extravasation: Dopamine Hydrochloride in 5% Dextrose Injection, USP should be infused into a large vein whenever possible to prevent the possibility of infiltration of perivascular tissue adjacent to the infusion site. Extravasation may cause necrosis and sloughing of surrounding tissue. Large veins of the antecubital fossa are preferred to veins of the dorsum of the hand or ankle. Administration into an umbilical arterial catheter is not recommended. Less suitable infusion sites should be used only when larger veins are unavailable and the patient's condition requires immediate attention. The physician should switch to a more suitable site as soon as possible and the infusion site in use should be continuously monitored for free flow.
IMPORTANT ─ Antidote for Peripheral Ischemia: To prevent sloughing and necrosis in ischemic areas, the area should be infiltrated as soon as possible with 10 to 15 mL of 0.9% Sodium Chloride Injection, USP, containing from 5 to 10 mg of Regitine® (brand of phentolamine) an adrenergic blocking agent. Pediatric dosage of Regitine® should be 0.1 ─ 0.2 mg/kg up to a maximum of 10 mg per dose. A syringe with a fine hypodermic needle should be used, and the solution liberally infiltrated throughout the ischemic area. Sympathetic blockade with phentolamine causes immediate and conspicuous local hyperemic changes if the area is infiltrated within 12 hours. Therefore, phentolamine should be given as soon as possible after the extravasation is noted.
Laboratory Tests: Infusion of dopamine suppresses pituitary secretion of thyroid–stimulating hormone, growth hormone, and prolactin.
Weaning: When discontinuing the infusion, it may be necessary to gradually decrease the dose of dopamine HCl while expanding blood volume with intravenous fluids. Sudden cessation may result in marked hypotension.
Drug Interactions: Cyclopropane or halogenated hydrocarbon anesthetics increase cardiac autonomic irritability and may sensitize the myocardium to the action of certain intravenously administered catecholamines, such as dopamine. This interaction appears to be related both to pressor activity and to the β-adrenergic stimulating properties of these catecholamines, and may produce ventricular arrhythmias and hypertension. Therefore, EXTREME CAUTION should be exercised when administering dopamine HCl to patients receiving cyclopropane or halogenated hydrocarbon anesthetics. Results of studies in animals indicate that dopamine-induced ventricular arrhythmias during anesthesia can be reversed by propranolol.
Because dopamine is metabolized by monoamine oxidase (MAO), inhibition of this enzyme prolongs and potentiates the effect of dopamine. Patients who have been treated with MAO inhibitors within two to three weeks prior to the administration of dopamine should receive initial doses of dopamine hydrochloride no greater than one-tenth (1/10) of the usual dose.
Concurrent administration of low-dose dopamine HCl and diuretic agents may produce an additive or potentiating effect on urine flow.
Tricyclic antidepressants may potentiate the cardiovascular effects of adrenergic agents.
Cardiac effects of dopamine are antagonized by β-adrenergic blocking agents, such as propranolol and metoprolol. The peripheral vasoconstriction caused by high doses of dopamine HCl is antagonized by α-adrenergic blocking agents. Dopamine-induced renal and mesenteric vasodilation is not antagonized by either α- or β-adrenergic blocking agents.
Butyrophenones (such as haloperidol) and phenothiazines can suppress the dopaminergic renal and mesenteric vasodilation induced with low-dose dopamine infusion.
The concomitant use of vasopressors, vasoconstricting agents (such as ergonovine) and some oxytocic drugs may result in severe hypertension.
Administration of phenytoin to patients receiving dopamine HCl has been reported to lead to hypotension and bradycardia. It is suggested that in patients receiving dopamine HCl, alternatives to phenytoin should be considered if anticonvulsant therapy is needed.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long term animal studies have not been performed to evaluate the carcinogenic potential of dopamine HCl.
Dopamine HCl at doses approaching maximal solubility showed no clear genotoxic potential in the Ames test. Although there was a reproducible dose-dependent increase in the number of revertant colonies with strains TA100 and TA98, both with and without metabolic activation, the small increase was considered inconclusive evidence of mutagenicity. In the L5178Y TK+/- mouse lymphoma assay, dopamine HCl at the highest concentrations used of 750 μg/mL without metabolic activation, and 3000 μg/mL with activation, was toxic and associated with increases in mutant frequencies when compared to untreated and solvent controls; at the lower concentrations no increases over controls were noted.
No clear evidence of clastogenic potential was reported in the in vivo mouse or male rat bone marrow micronucleus test when the animals were treated intravenously with up to 224 mg/kg and 30 mg/kg of dopamine HCl, respectively.
Pregnancy: Teratogenic Effects: Teratogenicity studies in rats and rabbits at dopamine HCl dosages up to 6 mg/kg/day intravenously during organogenesis produced no detectable teratogenic or embryotoxic effects, although maternal toxicity consisting of mortalities, decreased body weight gain, and pharmacotoxic signs were observed in rats. In a published study, dopamine HCl administered at 10 mg/kg subcutaneously for 30 days, markedly prolonged metestrus and increased mean pituitary and ovary weights in female rats. Similar administration to pregnant rats throughout gestation or for 5 days starting on gestation day 10 or 15 resulted in decreased body weight gains, increased mortalities and slight increases in cataract formation among the offspring. There are no adequate and well-controlled studies in pregnant women, and it is not known if dopamine HCl crosses the placental barrier. Dopamine HCl should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery: In obstetrics, if vasopressor drugs are used to correct hypotension or are added to a local anesthetic solution the interaction with some oxytocic drugs may cause severe hypertension.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dopamine HCl is administered to a nursing mother.
Pediatric Use: Dopamine infusions have been used in patients of every age from birth onwards. There are scattered reports of infusion rates in neonates up to 125 mcg/kg/min, but most reports in pediatric patients describe dosing that is similar (on a mcg/kg/min basis) to that used in adults. Except for vasoconstrictive effects caused by inadvertent infusion of dopamine into the umbilical artery, adverse effects unique to the pediatric population have not been identified, nor have adverse effects identified in adults been found to be more common in pediatric patients.
Geriatric Use: Clinical studies of dopamine injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Cardiovascular System
ventricular arrhythmia
atrial fibrillation
ectopic beats
tachycardia
anginal pain
palpitation
cardiac conduction abnormalities
widened QRS complex
bradycardia
hypotension
hypertension
vasoconstrictionRespiratory System
dyspneaGastrointestinal System
nausea
vomitingMetabolic/Nutritional System
azotemiaCentral Nervous System
headache
anxietyDermatological System
piloerectionOther
Gangrene of the extremities has occurred when high doses were administered for prolonged periods or in patients with occlusive vascular disease receiving low doses of dopamine HCl. -
OVERDOSAGE
In the case of accidental overdosage, as evidenced by excessive blood pressure elevation, reduce rate of infusion, or temporarily discontinue administration of the drug until patient's condition stabilizes. Since dopamine's duration of action is quite short, no additional remedial measures are usually necessary. If these measures fail to stabilize the patient's condition, consider using an alpha-adrenergic blocking agent (e.g., phentolamine).
-
DOSAGE AND ADMINISTRATION
Do NOT administer if solution is darker than slightly yellow or discolored in any other way. Do NOT administer unless solution is clear and container is undamaged. Discard unused portion.
Dextrose solutions without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
Do NOT add sodium bicarbonate or other alkalinizing substance, since dopamine is inactivated in alkaline solution.
Dopamine Hydrochloride in 5% Dextrose Injection should be infused into a large vein whenever possible to prevent the infiltration of perivascular tissue adjacent to the infusion site. Extravasation may cause necrosis and sloughing of the surrounding tissue. Large veins of the antecubital fossa are preferred to veins of the dorsum of the hand or ankle. Less suitable infusion sites should be used only when larger veins are unavailable and the patient's condition requires immediate attention. The physician should switch to a more suitable site as soon as possible and the infusion site in use should be continuously monitored for free flow.
The less concentrated 800 mcg/mL solution may be preferred when fluid expansion is not a problem. The more concentrated 1600 mcg/mL or 3200 mcg/mL solutions, may be preferred in patients with fluid retention or when a slower rate of infusion is desired.
Rate of Administration: Administration into an umbilical artery catheter is not recommended.
Dopamine in 5% Dextrose Injection should not be infused through ordinary intravenous apparatus, regulated only by gravity and mechanical clamps. Only an infusion pump, preferably a volumetric pump, should be used.
Each patient must be individually titrated to the desired hemodynamic or renal response to dopamine.
In titrating to the desired increase in systolic blood pressure, the optimum dosage rate for renal response may be exceeded, thus necessitating a reduction in rate after the hemodynamic condition is stabilized.
If a disproportionate rise in diastolic pressure (i.e., a marked decrease in pulse pressure) is observed in patients receiving dopamine, the infusion rate should be decreased and the patient observed carefully for further evidence of predominant vasoconstrictor activity, unless such an effect is desired.
Administration rates greater than 50 mcg/kg/min have safely been used in adults in advanced circulatory decompensation states. If unnecessary fluid expansion is of concern, adjustment of drug concentration may be preferred over increasing the flow rate of a less concentrated dilution.
When discontinuing the infusion, it may be necessary to gradually decrease the dose of dopamine HCl while expanding the blood volume with intravenous fluids to prevent the development of marked hypotension.
Suggested Regimen:
- 1. When appropriate, increase blood volume with whole blood or plasma until central venous pressure is 10 to 15 cm H2O or pulmonary wedge pressure is 14 to 18 mm Hg.
- 2.
Begin infusion of dopamine hydrochloride solution at doses of 2 to 5 mcg/kg/min in adult or pediatric patients who are likely to respond to modest increments of heart force and renal perfusion.
In more seriously ill patients, begin infusion of dopamine hydrochloride at doses of 5 mcg/kg/min and increase gradually, using 5 to 10 mcg/kg/min increments, up to a rate of 20 to 50 mcg/kg/min as needed. If doses in excess of 50 mcg/kg/min are required, check urine output frequently. Should urinary flow begin to decrease in the absence of hypotension, reduction of dopamine dosage should be considered. More than 50% of adult patients have been satisfactorily maintained on doses less than 20 mcg/kg/min.
In patients who do not respond to these doses with adequate arterial pressures or urine flow, additional increments of dopamine may be given in an effort to produce an appropriate arterial pressure and central perfusion. - 3.
Treatment of all patients requires constant evaluation of therapy in terms of blood volume, augmentation of cardiac contractility, urine flow, cardiac output, blood pressure, and distribution of peripheral perfusion.
Dosage of dopamine should be adjusted according to the patient's response. Diminution of established urine flow rate, increasing tachycardia or development of new dysrhythmias are reasons to consider decreasing or temporarily suspending the dosage. - 4. As with all potent intravenously administered drugs, care should be taken to control the rate of infusion so as to avoid inadvertent administration of a bolus of the drug.
800 mcg/mL Dosing Chart for Dopamine (mL/hr) Infusion Rate Infusion rate
(mcg/kg/min)
Patient Body Weight (kg)
10
20
30
40
50
60
70
80
90
100
2.5
1.9
3.8
5.6
7.5
9.4
11.3
13.1
15
16.9
18.8
5
3.8
7.5
11.3
15
18.8
22.5
26.3
30
33.8
37.5
10
7.5
15
22.5
30
37.5
45
52.5
60
67.5
75
15
11.3
22.5
33.8
45
56.3
67.5
78.8
90
101.3
112.5
20
15
30
45
60
75
90
105
120
135
150
25
18.8
37.5
56.3
75
93.8
112.5
131.3
150
168.8
187.5
30
22.5
45
67.5
90
112.5
135
157.5
180
202.5
225
35
26.3
52.5
78.8
105
131.3
157.5
183.8
210
236.3
262.5
40
30
60
90
120
150
180
210
240
270
300
45
33.8
67.5
101.3
135
168.8
202.5
236.3
270
303.8
337.5
50
37.5
75
112.5
150
187.5
225
262.5
300
337.5
375
1600 mcg/mL Dosing Chart for Dopamine (mL/hr) Infusion Rate Infusion rate
Patient Body Weight (kg)
(mcg/kg/min)
10
20
30
40
50
60
70
80
90
100
2.5
0.9
1.9
2.8
3.8
4.7
5.6
6.6
7.5
8.4
9.4
5
1.9
3.8
5.6
7.5
9.4
11.3
13.1
15
16.9
18.8
10
3.8
7.5
11.3
15
18.8
22.5
26.3
30
33.8
37.5
15
5.6
11.3
16.9
22.5
28.1
33.8
39.4
45
50.6
56.3
20
7.5
15
22.5
30
37.5
45
52.5
60
67.5
75
25
9.4
18.8
28.1
37.5
46.9
56.3
65.6
75
84.4
93.8
30
11.3
22.5
33.8
45
56.3
67.5
78.8
90
101.3
112.5
35
13.1
26.3
39.4
52.5
65.6
78.8
91.9
105
118.1
131.3
40
15
30
45
60
75
90
105
120
135
150
45
16.9
33.8
50.6
67.5
84.4
101.3
118.1
135
151.9
168.8
50
18.8
37.5
56.3
75
93.8
112.5
131.3
150
168.8
187.5
3200 mcg/mL Dosing Chart for Dopamine (mL/hr) Infusion Rate Infusion rate
Patient Body Weight (kg)
(mcg/kg/min)
10
20
30
40
50
60
70
80
90
100
2.5
0.5
0.9
1.4
1.9
2.3
2.8
3.3
3.8
4.2
4.7
5
0.9
1.9
2.8
3.8
4.7
5.6
6.6
7.5
8.4
9.4
10
1.9
3.8
5.6
7.5
9.4
11.3
13.1
15
16.9
18.8
15
2.8
5.6
8.4
11.3
14.1
16.9
19.7
22.5
25.3
28.1
20
3.8
7.5
11.3
15
18.8
22.5
26.3
30
33.8
37.5
25
4.7
9.4
14.1
18.8
23.4
28.1
32.8
37.5
42.2
46.9
30
5.6
11.3
16.9
22.5
28.1
33.8
39.4
45
50.6
56.3
35
6.6
13.1
19.7
26.3
32.8
39.4
45.9
52.5
59.1
65.6
40
7.5
15
22.5
30
37.5
45
52.5
60
67.5
75
45
8.4
16.9
25.3
33.8
42.2
50.6
59.1
67.5
75.9
84.4
50
9.4
18.8
28.1
37.5
46.9
56.3
65.6
75
84.4
93.8
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
INSTRUCTIONS FOR USE
To Open
Tear outer wrap at notch and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Preparation for Administration
(Use aseptic technique)- 1. Close flow control clamp of administration set.
- 2. Remove cover from outlet port at bottom of container.
- 3. Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: See full directions on administration set carton.
- 4. Suspend container from hanger.
- 5. Squeeze and release drip chamber to establish proper fluid level in chamber.
- 6. Open flow control clamp and clear air from set. Close clamp.
- 7. Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- 8. Regulate rate of administration with an infusion pump, preferably a volumetric pump.
WARNING: Do not use flexible container in series connections.
-
HOW SUPPLIED
Dopamine Hydrochloride in 5% Dextrose Injection, USP is supplied in 250 and 500 mL LifeCare flexible containers as follows:
Unit of Sale Total Strength/Total Volume
(Concentration)NDC: 0409-7808-22
12 in a carton200 mg/250 mL
(800 mcg/mL)NDC: 0409-7808-24
12 in a carton400 mg/500 mL
(800 mcg/mL)NDC: 0409-7809-22
12 in a carton400 mg/250 mL
(1600 mcg/mL)NDC: 0409-7809-24
12 in a carton800 mg/500 mL
(1600 mcg/mL)NDC: 0409-7810-22
12 in a carton800 mg/250 mL
(3200 mcg/mL)Avoid contact with alkalies (including sodium bicarbonate), oxidizing agents or iron salts.
Do not use the injection if it is darker than slightly yellow or discolored in any other way.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA

LAB-1153-1.0
Revised: 03/2018
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - WR-1542
TO OPEN — TEAR AT NOTCH
Each 100 mL contains dopamine hydrochloride 80 mg; dextrose,
hydrous 5 g in water for injection; sodium metabisulfite added 50 mg.
May contain hydrochloric acid and/or sodium hydroxide for pH
adjustment.
261 mOsmol/liter (calc.)
ph 3.8 (2.5 to 4.5).Single-dose container. Discard unused portion. For intravenous use.
Usual dosage: see insert. WARNING: CONTAINS SULFITES.Drug additives should not be made to this solution. The overwrap is a
moisture and oxygen barrier. Do not remove unit from overwrap until
ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged or if solution is darker than slightly yellow
or discolored in any other way. Use unit promptly when overwrap is
opened. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room
Temperature.] Protect from freezing. See insert. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.Rx only
250 mL
NDC: 0409-7808-11DOPAMINE HCl
in 5% Dextrose Injection, USP200 mg/250 mL
(800 mcg/mL)Single-dose container
F WR-1542
Distributed by
Hospira, Inc., Lake Forest, IL 60045 USAHospira

-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - IM-4353
250 mL
NDC: 0409-7808-11DOPAMINE HCl
in 5% Dextrose Injection, USP200 mg/250 mL
(800 mcg/mL)EACH 100 mL CONTAINS DOPAMINE
HYDROCHLORIDE 80 mg; DEXTROSE,
HYDROUS 5 g IN WATER FOR
INJECTION; SODIUM METABISULFITE
ADDED 50 mg. MAY CONTAIN
HYDROCHLORIC ACID AND/OR
SODIUM HYDROXIDE FOR pH
ADJUSTMENT. 261 mOsmol/ LITER
(CALC.) pH 3.8 (2.5 to 4.5). SINGLE-
DOSE CONTAINER. DISCARD UNUSED
PORTION. FOR INTRAVENOUS USE.
USUAL DOSAGE: SEE INSERT.
WARNING: CONTAINS SULFITES.DRUG ADDITIVES SHOULD NOT
BE MADE TO THIS SOLUTION.STERILE NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR
AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES
CONNECTIONS.Rx ONLY
7
OTHERHospira
IM-4353
DISTRIBUTED BY HOSPIRA, INC., LAKE FOREST, IL 60045 USA

-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - WR-1541
TO OPEN — TEAR AT NOTCH
Each 100 mL contains dopamine hydrochloride 80 mg; dextrose,
hydrous 5 g in water for injection; sodium metabisulfite added 50 mg.
May contain hydrochloric acid and/or sodium hydroxide for pH
adjustment.
261 mOsmol/liter (calc.)
ph 3.8 (2.5 to 4.5).Single-dose container. Discard unused portion. For intravenous use.
Usual dosage: see insert. WARNING: CONTAINS SULFITES.Drug additives should not be made to this solution. The overwrap is a
moisture and oxygen barrier. Do not remove unit from overwrap until
ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged or if solution is darker than slightly yellow
or discolored in any other way. Use unit promptly when overwrap is
opened. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room
Temperature.] Protect from freezing. See insert. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.Rx only
500 mL
NDC: 0409-7808-31DOPAMINE HCl
in 5% Dextrose Injection, USP400 mg/500 mL
(800 mcg/mL)Single-dose container
F WR-1541
Distributed by
Hospira, Inc., Lake Forest, IL 60045 USAHospira

-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - IM-4354
500 mL
NDC: 0409-7808-31DOPAMINE HCl
in 5% Dextrose Injection, USP400 mg/500 mL
(800 mcg/mL)EACH 100 mL CONTAINS DOPAMINE
HYDROCHLORIDE 80 mg; DEXTROSE,
HYDROUS 5 g IN WATER FOR INJECTION;
SODIUM METABISULFITE ADDED 50 mg.
MAY CONTAIN HYDROCHLORIC ACID
AND/OR SODIUM HYDROXIDE FOR pH
ADJUSTMENT.
261 mOsmol/ LITER (CALC.) pH 3.8
(2.5 to 4.5). SINGLE-DOSE CONTAINER.
DISCARD UNUSED PORTION. FOR
INTRAVENOUS USE. USUAL DOSAGE: SEE
INSERT. WARNING: CONTAINS SULFITES.DRUG ADDITIVES SHOULD NOT
BE MADE TO THIS SOLUTION.STERILE NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED. MUST NOT
BE USED IN SERIES CONNECTIONS.Rx ONLY
7
OTHERHospira
IM-4354
DISTRIBUTED BY HOSPIRA, INC., LAKE FOREST, IL 60045 USA

-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - WR-1540
TO OPEN — TEAR AT NOTCH
Each 100 mL contains dopamine hydrochloride 160 mg; dextrose,
hydrous 5 g in water for injection; sodium metabisulfite added 50 mg.
May contain hydrochloric acid and/or sodium hydroxide for pH
adjustment.
269 mOsmol/liter (calc.)
ph 3.8 (2.5 to 4.5).Single-dose container. Discard unused portion. For intravenous use.
Usual dosage: see insert. WARNING: CONTAINS SULFITES.Drug additives should not be made to this solution. The overwrap is a
moisture and oxygen barrier. Do not remove unit from overwrap until
ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged or if solution is darker than slightly yellow
or discolored in any other way. Use unit promptly when overwrap is
opened. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room
Temperature.] Protect from freezing. See insert. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.Rx only
250 mL
NDC: 0409-7809-11DOPAMINE HCl
in 5% Dextrose Injection, USP400 mg/250 mL
(1,600 mcg/mL)Single-dose container
F WR-1540
Distributed by
Hospira, Inc., Lake Forest, IL 60045 USAHospira

-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - IM-4355
250 mL
NDC: 0409-7809-11DOPAMINE HCl
in 5% Dextrose Injection, USP400 mg/250 mL
(1,600 mcg/mL)EACH 100 mL CONTAINS
DOPAMINE HYDROCHLORIDE
160 mg; DEXTROSE, HYDROUS 5 g IN
WATER FOR INJECTION; SODIUM
METABISULFITE ADDED 50 mg. MAY
CONTAIN HYDROCHLORIC ACID
AND/OR SODIUM HYDROXIDE FOR
pH ADJUSTMENT.
269 mOsmol/LITER (CALC.) pH 3.8
(2.5 to 4.5). SINGLE-DOSE
CONTAINER. DISCARD UNUSED
PORTION. FOR INTRAVENOUS
USE. USUAL DOSAGE: SEE INSERT.
WARNING: CONTAINS SULFITES.DRUG ADDITIVES SHOULD NOT
BE MADE TO THIS SOLUTION.STERILE NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR
AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES
CONNECTIONS.Rx ONLY
7
OTHERHospira
IM-4355
DISTRIBUTED BY HOSPIRA, INC., LAKE FOREST, IL 60045 USA

-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - WR-1543
TO OPEN — TEAR AT NOTCH
Each 100 mL contains dopamine hydrochloride 160 mg; dextrose,
hydrous 5 g in water for injection; sodium metabisulfite added 50 mg.
May contain hydrochloric acid and/or sodium hydroxide for pH
adjustment.
269 mOsmol/liter (calc.)
ph 3.8 (2.5 to 4.5).Single-dose container. Discard unused portion. For intravenous use.
Usual dosage: see insert. WARNING: CONTAINS SULFITES.Drug additives should not be made to this solution. The overwrap is a
moisture and oxygen barrier. Do not remove unit from overwrap until
ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged or if solution is darker than slightly yellow
or discolored in any other way. Use unit promptly when overwrap is
opened. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room
Temperature.] Protect from freezing. See insert. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.Rx only
500 mL
NDC: 0409-7809-31DOPAMINE HCl
in 5% Dextrose Injection, USP800 mg/500 mL
(1,600 mcg/mL)Single-dose container
F WR-1543
Distributed by
Hospira, Inc., Lake Forest, IL 60045 USAHospira

-
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - IM-4356
500 mL
NDC: 0409-7809-31DOPAMINE HCl
in 5% Dextrose Injection, USP800 mg/500 mL
(1,600 mcg/mL)EACH 100 mL CONTAINS DOPAMINE
HYDROCHLORIDE 160 mg; DEXTROSE,
HYDROUS 5 g IN WATER FOR INJECTION;
SODIUM METABISULFITE ADDED 50 mg.
MAY CONTAIN HYDROCHLORIC ACID
AND/OR SODIUM HYDROXIDE FOR pH
ADJUSTMENT.
269 mOsmol/LITER (CALC.) pH 3.8 (2.5 to
4.5). SINGLE-DOSE CONTAINER. DISCARD
UNUSED PORTION. FOR INTRAVENOUS
USE. USUAL DOSAGE: SEE INSERT.
WARNING: CONTAINS SULFITES.DRUG ADDITIVES SHOULD NOT
BE MADE TO THIS SOLUTION.STERILE NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED. MUST NOT
BE USED IN SERIES CONNECTIONS.Rx ONLY
7
OTHERHospira
IM-4356
DISTRIBUTED BY HOSPIRA, INC., LAKE FOREST, IL 60045 USA

-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - WR-1544
TO OPEN — TEAR AT NOTCH
Each 100 mL contains dopamine hydrochloride 320 mg; dextrose,
hydrous 5 g in water for injection; sodium metabisulfite added 50 mg.
May contain hydrochloric acid and/or sodium hydroxide for pH
adjustment.
286 mOsmol/liter (calc.)
ph 3.8 (2.5 to 4.5).Single-dose container. Discard unused portion. For intravenous use.
Usual dosage: see insert. WARNING: CONTAINS SULFITES.Drug additives should not be made to this solution. The overwrap is a
moisture and oxygen barrier. Do not remove unit from overwrap until
ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged or if solution is darker than slightly yellow
or discolored in any other way. Use unit promptly when overwrap is
opened. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room
Temperature.] Protect from freezing. See insert. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.Rx only
250 mL
NDC: 0409-7808-11DOPAMINE HCl
in 5% Dextrose Injection, USP800 mg/250 mL
(3,200 mcg/mL)Single-dose container
F WR-1544
Distributed by
Hospira, Inc., Lake Forest, IL 60045 USAHospira

-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - IM-4357
250 mL
NDC: 0409-7810-11DOPAMINE HCl
in 5% Dextrose Injection, USP800 mg/250 mL
(3,200 mcg/mL)EACH 100 mL CONTAINS DOPAMINE
HYDROCHLORIDE 320 mg; DEXTROSE,
HYDROUS 5 g IN WATER FOR
INJECTION; SODIUM METABISULFITE
ADDED 50 mg. MAY CONTAIN
HYDROCHLORIC ACID AND/OR
SODIUM HYDROXIDE FOR pH
ADJUSTMENT. 286 mOsmol/LITER
(CALC.) pH 3.8 (2.5 to 4.5). SINGLE-
DOSE CONTAINER. DISCARD UNUSED
PORTION. FOR INTRAVENOUS USE.
USUAL DOSAGE: SEE INSERT.
WARNING: CONTAINS SULFITES.DRUG ADDITIVES SHOULD NOT
BE MADE TO THIS SOLUTION.STERILE NONPYROGENIC.
USE ONLY IF SOLUTION IS CLEAR
AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES
CONNECTIONS.Rx ONLY
7
OTHERIM-4357
Hospira
DISTRIBUTED BY HOSPIRA, INC., LAKE FOREST, IL 60045 USA

-
INGREDIENTS AND APPEARANCE
DOPAMINE HYDROCHLORIDE IN DEXTROSE
dopamine hydrochloride in dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-7808 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOPAMINE HYDROCHLORIDE (UNII: 7L3E358N9L) (DOPAMINE - UNII:VTD58H1Z2X) DOPAMINE HYDROCHLORIDE 0.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) WATER (UNII: 059QF0KO0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-7808-22 12 in 1 CARTON 06/21/2005 10/01/2017 1 NDC: 0409-7808-11 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0409-7808-24 12 in 1 CARTON 08/08/2005 02/01/2018 2 NDC: 0409-7808-31 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018826 06/21/2005 02/01/2018 DOPAMINE HYDROCHLORIDE IN DEXTROSE
dopamine hydrochloride in dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-7809 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOPAMINE HYDROCHLORIDE (UNII: 7L3E358N9L) (DOPAMINE - UNII:VTD58H1Z2X) DOPAMINE HYDROCHLORIDE 1.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) SODIUM METABISULFITE (UNII: 4VON5FNS3C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-7809-22 12 in 1 CARTON 04/30/2005 1 NDC: 0409-7809-11 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0409-7809-24 12 in 1 CARTON 10/14/2005 2 NDC: 0409-7809-31 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018826 04/30/2005 DOPAMINE HYDROCHLORIDE IN DEXTROSE
dopamine hydrochloride in dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0409-7810 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOPAMINE HYDROCHLORIDE (UNII: 7L3E358N9L) (DOPAMINE - UNII:VTD58H1Z2X) DOPAMINE HYDROCHLORIDE 3.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) WATER (UNII: 059QF0KO0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-7810-22 12 in 1 CARTON 08/08/2005 1 NDC: 0409-7810-11 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018826 08/08/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-7808, 0409-7809, 0409-7810) , LABEL(0409-7808, 0409-7809, 0409-7810) , MANUFACTURE(0409-7808, 0409-7809, 0409-7810) , PACK(0409-7808, 0409-7809, 0409-7810) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-7808, 0409-7809, 0409-7810)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.