MENCAPS- capsaicin and menthol patch
MenCaps by
Drug Labeling and Warnings
MenCaps by is a Otc medication manufactured, distributed, or labeled by Alexso Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients:
- Purpose
- Active ingredients:
- Purpose
- Uses:
-
Directions:
Adults and children 12 years and over: Apply 1 patch to the affected area of intact skin up to 3 times a day. Do not leave patch on for more than 8 hours at a time.
Instructions for Use:
- Clean and dry the affected area.
- Open pouch and remove one patch.
-
Remove any protective film and apply directly to affected area of pain.
Apply immediately after removal from the protective envelope. - Wash hands with soap and water after handling the patches.
- Reseal pouch containing unused patches after each use. Do not store patch outside the sealed envelope.
- Discontinue use at least 1 hour before a bath or shower and do not use immediately after a bath or shower.
- Fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them.
-
Warnings:
- For external use only. Use only as directed or by a health professional.
-
Do not use: On wounds, cuts, damaged or infected skin.
- On eyes, mouth, genitals, or any other mucus membranes. - Stop and consult your physician if pregnant or if pain worsens
- Allergy Alert: if you are allergic to any ingredients of this product or chili peppers contact a doctor before use.
- Keep out of reach of children.
- Other Ingredients:
- Questions or Comments
-
PACKAGE INSERT
Patient Information
(updated 6/2015)
Read the patient information sheet provided before you start using this medication and each time you get a refill. If you have any questions, please consult your doctor or pharmacist. Inform your doctor if your condition does not improve or if it worsens.
This information may not include all of the information needed to use MenCaps Patch® safely and effectively.
For Topical Use Only
What is MenCaps Patch®?
This is a topical patch consisting of the topical analgesic, menthol, and the topical analgesic, capsaicin extract.
What is MenCaps Patch® used for?
The patch is applied to the skin assisting patients in the management of mild to moderate acute or moderate aches or pain of muscles and joints associated with arthritis, backache, strains and sprains. The diagnosis of acute or chronic topical pain can be made relatively simply by the diagnostic practitioner, however uncovering the underlying pathology manifesting as pain and discomfort is often more difficult.
The combination of capsaicin and menthol is a highly effective combination of working synergistically to ease the discomfort while the root cause is being managed and treated by your healthcare team.
DO NOT USE:
- If you are allergic to any of the ingredients in this product
- On wounds or damaged skin
- With a heating pad
- Concurrently with any other external pain-relieving products
What are the possible side effects with MenCaps Patch®
- Common (≥10%): itching, redness or flaking of the skin following application (note: the majority of patients experience no significant adverse events following patch application).
- NOTE: serious side effects are, in general, related to accidental toxicity of medication by applying considerably more than directed by your doctor or pharmacist
- Tell your healthcare provider about all the medicines you take. This includes prescription and nonprescription medicines, vitamins, and herbal supplements
WARNINGS:
- 1. Do not use:
- On wounds, cuts, damaged or infected skin
- On eyes, mouth, genitals, or other mucous membranes
- 2. Consult physician for children under 12 years of age
- 3. Stop and consult your prescriber if pain worsens
- 4. DISCONTINUE USE AT LEAST 1 HOURS BEFORE A BATH OR SHOWER AND DO NOT USE IMMEDIATELY AFTER A BATH OR SHOWER
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- Shortness of breath
- Swelling or numbness of the tongue or throat
- Severe headache or vomiting
- Dizziness or faintness
- Changes in vision or speech
This is not a complete list of the possible side effects. For more information, talk with your doctor or pharmacist. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DIRECTIONS FOR USE:
Adults and children 12 years and over: Apply 1 patch to the affected area of intact skin up to 3 times a day. MenCaps Patch® should not be left on for more than 8 hours at a time.
- 1. Clean and dry the affected area.
- 2. Open pouch and remove one patch.
- 3. Remove any protective film and apply directly to the painful area. Apply immediately after opening.
- 4. Wash hands with soap and water after handling the patches.
- 5. Reseal pouch containing unused patches after each use. Do not store patch outside the sealed envelope.
- 6. Fold used patches so that the adhesive side sticks to itself. Next, safely discard the used patches or pieces of cut patches where children and pets cannot get to them.
Children under 12 years of age:
- Consult a doctor.
General information about the safe and effective use
MenCaps Patch®Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use this product for another indication. Your doctor has prescribed this drug for you and you alone. Do not give this drug to anyone else, even if they have the same condition.
How should I store MenCaps Patch®
Store product at room temperature at 68°F to 77°F (20°C to 25°C). Keep away from heat and sunlight. Protect from excessive moisture. Discard product after expiration date posted on the product label.
DO NOT use the product after the expiration date printed on the box.
KEEP OUT OF REACH OF CHILDREN
This leaflet provides the most important information about MenCaps Patch®. If you would like more information, talk with your healthcare provider or pharmacist. There is additional information in the following sections intended for healthcare professionals.
What are the active ingredients in MenCaps Patch®?
The patch consists of 4.5% menthol and 0.0225% capsaicin.
INACTIVE INGREDIENTS:
Water Aloe Barbadesis Leaf (Aloe Vera Gel)
Glycerol EDTA Disodium salt
Sodium Polyacrylate Diazolidinyl Urea
Polysorbate 80 Methylparaben
Propylparaben Citrus Medica Limonum
Iodopropynyl Butylcarbamate (Lemon) Peel OilDESCRIPTION:
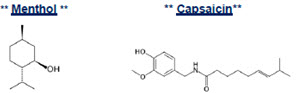
MenCaps Patch® is an OTC topical patch containing 15 articulated MenCaps patches (5 per re-sealable pouch x 3 pouches). Capsaicin is present in a 0.0225% concentration (w/w). It is chemically designated as (E)-N-[(4-Hydroxy-3-methoxyphenyl) methyl]-8- methylnon-6-enamide and has an empirical formula of C18H27NO3. The molecular weight of capsaicin is 305.41 g/mol. Menthol is present in a 4.5% concentration (w/w). The chemical name is (1R,2S,5R)-2- isopropyl-5-methylcyclohexanol. The empirical formula for menthol is C10H20O with a molecular weight of 156.27 g/mol.
The structural formulas are:
CLINICAL PHARMACOLOGY:
Capsaicin is an active component of chili peppers known clinically as a rubefacient. It acts as a counter-irritant by producing heat when in contact with human tissue. Mechanistically, capsaicin exerts its analgesic and anti-inflammatory effects by mimicking a burning sensation – whereby overwhelming the nerves by calcium influx, thereby rendering the nerves unable to signal a pain response for an extended period of time. With repeated exposure, the neurons become depleted of neurotransmitters –specifically substance P - leading to a reduction in the sensation of pain and the blockade of neurogenic inflammation. This process is reversed when the capsaicin is discontinued.
Menthol has local anesthetic and counterirritant qualities, and it also acts as a weak kappa (κ) opioid receptor agonist. Menthol acts to chemically trigger the cold-sensitive TRPM-8 receptors in the skin - responsible for the well-known cooling sensation it stimulates when applied directly to the skin. Menthol's analgesic properties are not fully understood, however they are mediated through a selective activation of κ-opioid receptors. Menthol also blocks voltage-sensitive sodium channels, reducing neural activity that may stimulate muscle tissue.
INDICATIONS AND USAGE:
MenCaps Patch® is a formulation used to assist patients for the temporary relief of minor aches & pains of muscles & joints associated with arthritis, simple backache, strains, bruises, and sprains. Peripheral neuropathies such as diabetic neuropathy or post herpetic neuralgia, may also be symptomatically improved using this patch if recommended by the healthcare team.
Other uses may be considered if deemed clinically relevant.
CONTRAINDICATIONS:
Known hypersensitivity to menthol, capsaicin, or any of the inactive ingredients listed above.
WARNINGS:
- 1. Do not use on wounds, cuts, damaged or infected skin
- 2. Do not use on eyes, mouth, genitals, or any other mucous membranes
- 3. Keep out of reach of children
PRECAUTIONS:
Because of the possibility of sedation, patients should be cautioned regarding the operation of serious machinery or automobiles, and activities made hazardous by decreased alertness.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
Nonclinical toxicity studies to determine the potential of this topical preparation to cause carcinogenicity or mutagenicity have not been performed. The effect of the product on fertility has not been evaluated in animals.
PREGNANCY:
The safety of MenCaps Patch® has not been established during pregnancy. There are no well-controlled studies in pregnant women.
PEDIATRIC / GERIATRIC USE:
Safety and effectiveness in pediatric and geriatric patients have not been established.
ADVERSE REACTIONS:
The most common adverse reactions are application site reactions, including dermatitis, itching or scaling. These tend to be dose-limiting and diminish with time.
Serious adverse experiences following the administration of MenCaps Patch® are similar in nature to those observed in other amide anesthetic-containing agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage, rapid absorption, or may result from hypersensitivity, idiosyncrasy, or a diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature.
OVERDOSAGE:
There have been no reports of over-dosage with MenCaps Patch®. Signs of overdosage would include vomiting, drowsiness, coma, respiratory depression, and seizures. In the case of an overdosage, discontinue the product immediately, treat the patient symptomatically, and institute supportive measures.
STORAGE:
Store at room temperature at 68°F to 77°F (20°C to 25°C). Keep away from heat or sunlight. Protect from excessive moisture. The product can be considered safe and effective to use when maintained under these recommended conditions within the posted expiration date.
HOW SUPPLIED:
MenCaps Patch® is supplied in the following dosage form:
- 15 Articulated Patches [(5 per Re-sealable Pouch) x 3]
Manufactured for : Alexso Inc
2317 Cotner Ave Los Angeles, CA 90064
www.alexso.com 888.495.6078NDC: 50488-1701-1 Size: 15 Patches
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MENCAPS
capsaicin and menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50488-1701 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.0225 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 4.5 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LEMON PEEL (UNII: 72O054U628) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50488-1701-1 3 in 1 BOX 07/01/2015 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/01/2015 Labeler - Alexso Inc (963338061) Registrant - Alexso Inc (963338061)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.