LORATADINE ODT- loratadine tablet, orally disintegrating
Loratadine ODT by
Drug Labeling and Warnings
Loratadine ODT by is a Otc medication manufactured, distributed, or labeled by Ahold U.S.A., Inc,, Aurohealth LLC, Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
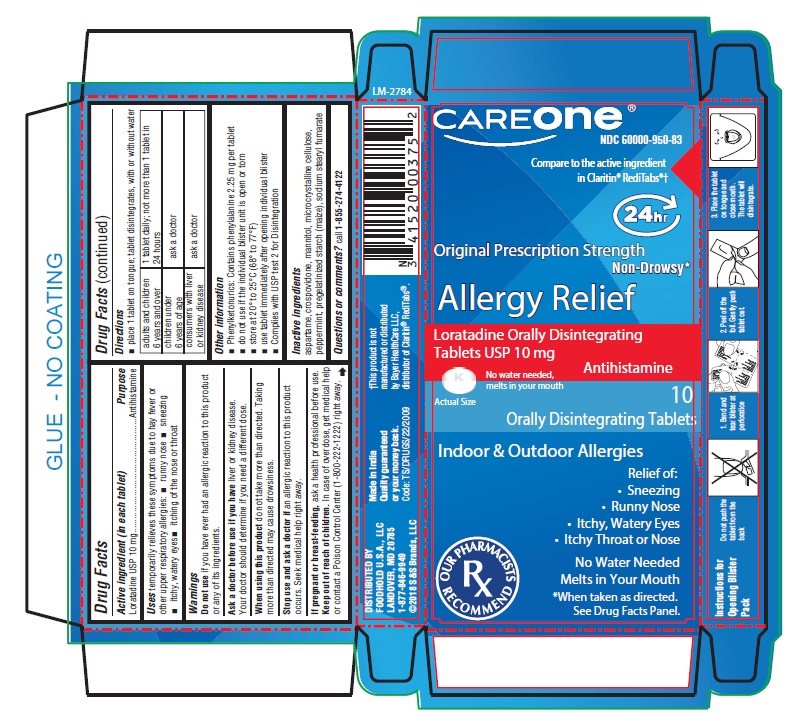
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg, Blister Carton 10 (1 X 10) Orally Disintegrating Tablets
CAREONE®
NDC: 60000-950-83
Compare to the active ingredient
in Claritin® RediTabs®†
24 hr
Original Prescription Strength
Non-Drowsy*
Allergy Relief
Loratadine Orally Disintegrating
Tablets USP 100 mg
No water needed,
melts in your mouth
Actual Size
10 Orally Disintegrating Tablets
Indoor & Outdoor Allergies
Relief of:- Sneezing
- Runny Nose
- Itchy, Water Eyes
- Itchy Throat or Nose
No Water Needed
Melts in Your Mouth
*When taken as directed.
See Drug Facts Panel.
-
INGREDIENTS AND APPEARANCE
LORATADINE ODT
loratadine tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60000-950 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PEPPERMINT (UNII: V95R5KMY2B) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color WHITE (White to Off-white) Score no score Shape ROUND (Biconvex) Size 8mm Flavor PEPPERMINT Imprint Code K;9 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60000-950-83 1 in 1 CARTON 04/11/2018 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208477 04/11/2018 Labeler - Ahold U.S.A., Inc, (188910863) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(60000-950) , MANUFACTURE(60000-950)
Trademark Results [Loratadine ODT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LORATADINE ODT 76601213 not registered Dead/Abandoned |
LEINER HEALTH SERVICES CORP. 2004-07-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.