ACTISEP- benzocaine, menthol, cetylpyridinium chloride solution
ACTISEP by
Drug Labeling and Warnings
ACTISEP by is a Otc medication manufactured, distributed, or labeled by Actipharma, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
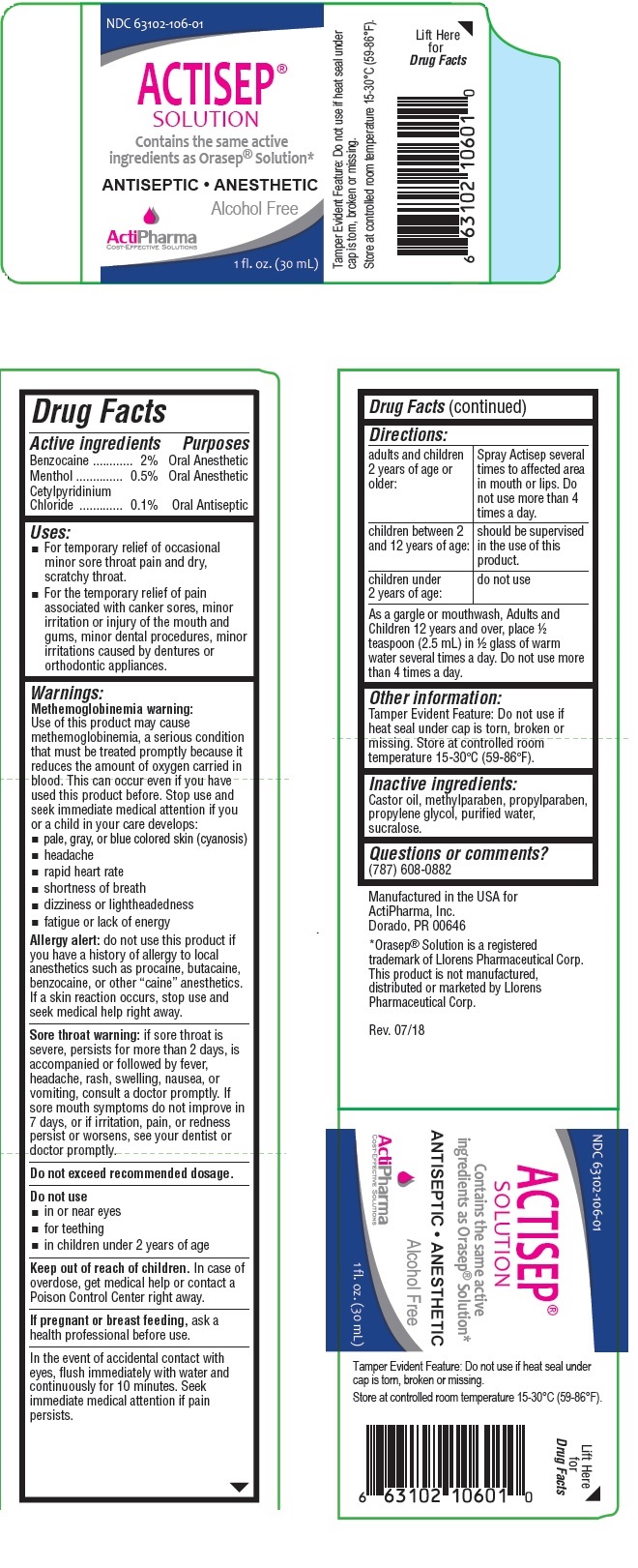
- Drug Facts
- Active ingredients
- Purposes
- Uses:
-
Warnings:
Methemoglobinemia warning:
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other “caine” anesthetics. If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persist or worsens, see your dentist or doctor promptly.
Do not exceed recommended dosage.
Do not use
- in or near eyes
- for teething
- in children under 2 years of age
If pregnant or breast feeding, ask a health professional before use.
In the event of accidental contact with eyes, flush immediately with water and continuously for 10 minutes. Seek immediate medical attention if pain persists.
-
Directions:
adults and children 2 years of age or older: Spray Actisep several times to affected area in mouth or lips. Do not use more than 4 times a day. children between 2 and 12 years of age: should be supervised in the use of this product. children under 2 years of age: do not use As a gargle or mouthwash, Adults and Children 12 years and over, place ½ teaspoon (2.5 mL) in ½ glass of warm
water several times a day. Do not use more than 4 times a day. - Inactive ingredients:
- Other information:
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
Contains the same active ingredients as Orasep® Solution*
ANTISEPTIC ANESTHETIC
Alcohol Free
Manufactured in the USA for
ActiPharma, Inc.
Dorado, PR 00646*Orasep® Solution is a registered trademark of Llorens Pharmaceutical Corp.
This product is not manufactured, distributed or marketed by Llorens Pharmaceutical Corp. - Packaging
-
INGREDIENTS AND APPEARANCE
ACTISEP
benzocaine, menthol, cetylpyridinium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63102-106 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 2 g in 100 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 0.5 g in 100 mL CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63102-106-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/28/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 12/28/2015 Labeler - Actipharma, Inc (079340948)
Trademark Results [ACTISEP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACTISEP 73686996 1522269 Dead/Cancelled |
GRANDICS, PETER 1987-09-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.