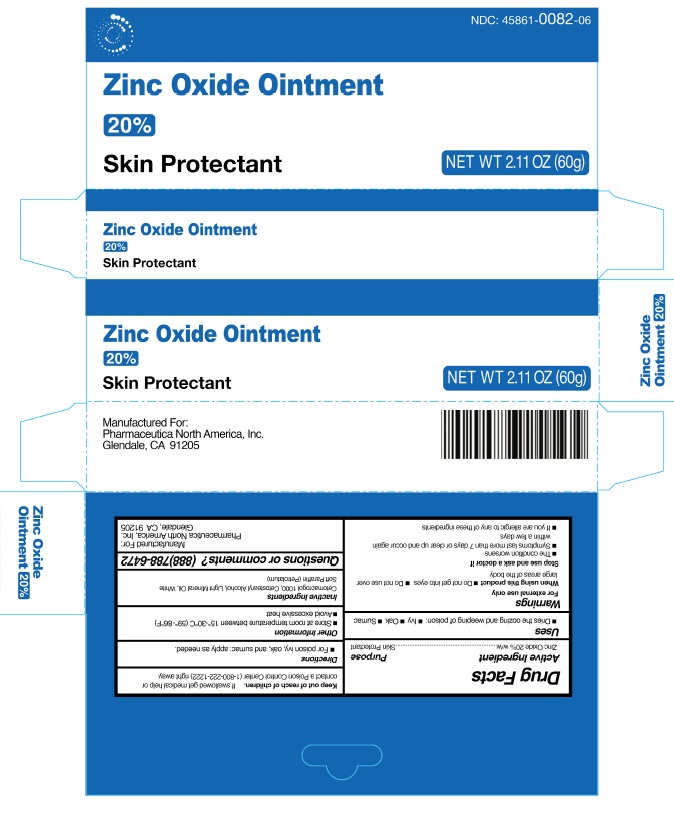
Zinc Oxide 20% by Pharmaceutica North America, Inc.
Zinc Oxide 20% by
Drug Labeling and Warnings
Zinc Oxide 20% by is a Otc medication manufactured, distributed, or labeled by Pharmaceutica North America, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZINC OXIDE 20%- zinc oxide ointment
Pharmaceutica North America, Inc.
----------
Warnings
For External Use Only
When using this product
Do not get into eyes
Do not use over large areas of the body
Stop use and ask a doctor if
The condition worsens
Symptoms last more than 7 days or clear up and occur again within a few days
If you are allergic to any of these ingredients
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
| ZINC OXIDE 20%
zinc oxide ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmaceutica North America, Inc. (962739699) |
Revised: 12/2025
Document Id: 471be45c-b7a7-ec9a-e063-6294a90ae569
Set id: 093302ce-ecf8-4df8-b671-a9ded4ab2c21
Version: 2
Effective Time: 20251229