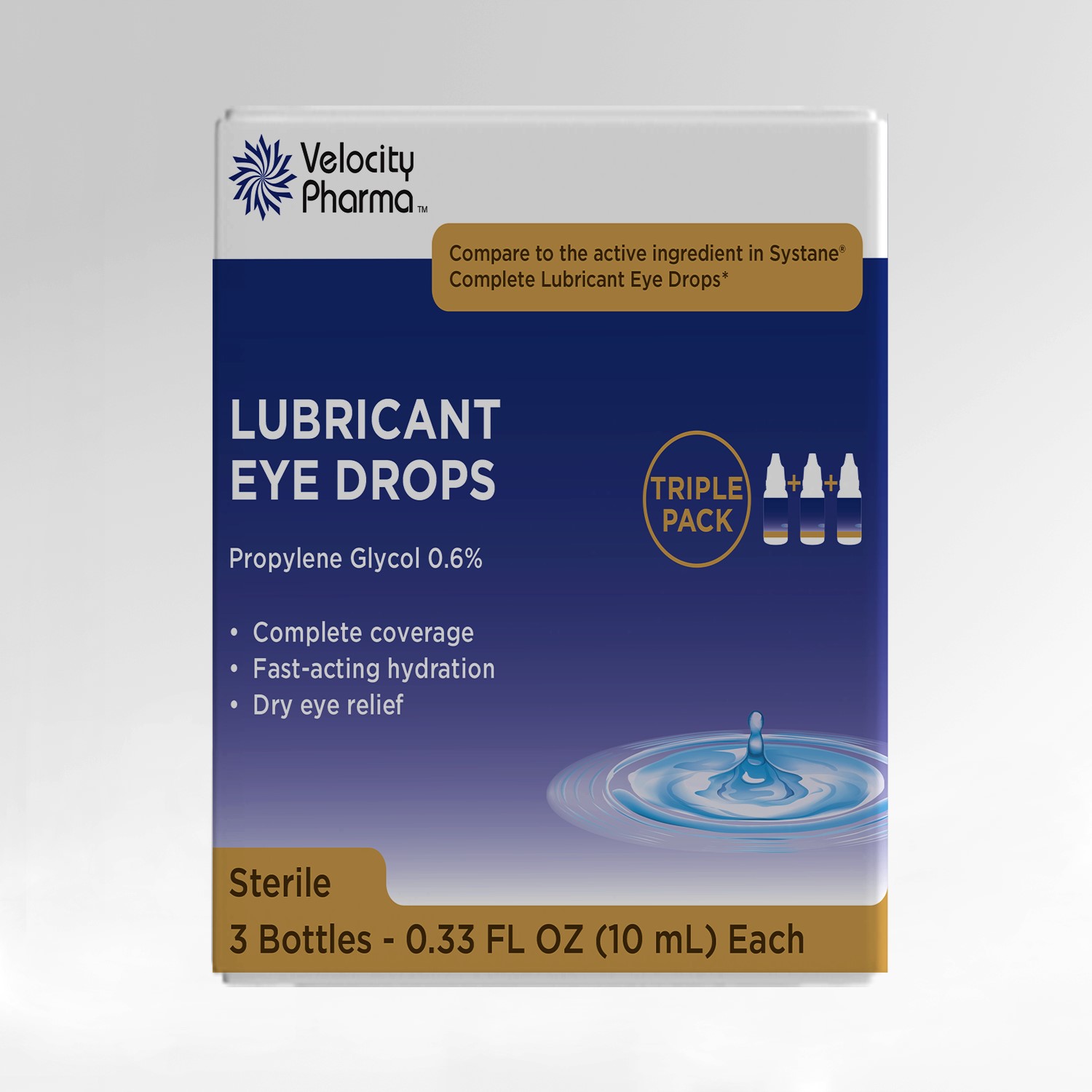
Lubricant Drops by VELOCITY PHARMA Lubricant Eye Drops
Lubricant Drops by
Drug Labeling and Warnings
Lubricant Drops by is a Otc medication manufactured, distributed, or labeled by VELOCITY PHARMA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LUBRICANT DROPS- propylene glycol solution/ drops
VELOCITY PHARMA
----------
Lubricant Eye Drops
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Directions
- shake well before using
- put 1 or 2 drops in the affected eye(s) as needed
- see side of carton for directions for use
Inactive Ingredients
benzalkonium chloride solution, boric acid, calcium chloride, magnesium chloride, potassium chloride, castor oil, disodium edetate, polyoxyl 40 hydrogenated castor oil, sodium borate, sodium hyaluronate, purified water. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
| LUBRICANT DROPS
propylene glycol solution/ drops |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - VELOCITY PHARMA (962198409) |
Revised: 1/2024
Document Id: 0fa85baf-513e-565a-e063-6394a90a326f
Set id: 0941224e-fbed-ac3e-e063-6394a90a70bf
Version: 3
Effective Time: 20240123
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.