KEYTRUDA QLEX- pembrolizumab and berahyaluronidase alfa-pmph injection, solution
KEYTRUDA QLEX by
Drug Labeling and Warnings
KEYTRUDA QLEX by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KEYTRUDA QLEX safely and effectively. See full prescribing information for KEYTRUDA QLEX.
KEYTRUDA QLEX™ (pembrolizumab and berahyaluronidase alfa-pmph) injection, for subcutaneous use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
KEYTRUDA QLEX is a combination of pembrolizumab, a programmed death receptor-1 (PD-1)-blocking antibody, and berahyaluronidase alfa, an endoglycosidase, indicated:
Melanoma
- for the treatment of adult patients with unresectable or metastatic melanoma. (1.1)
- for the adjuvant treatment of adult and pediatric patients 12 years and older with Stage IIB, IIC, or III melanoma following complete resection. (1.1)
Non-Small Cell Lung Cancer (NSCLC)
- in combination with pemetrexed and platinum chemotherapy, as first-line treatment of adult patients with metastatic nonsquamous NSCLC, with no EGFR or ALK genomic tumor aberrations. (1.2)
- in combination with carboplatin and either paclitaxel or paclitaxel protein-bound, as first-line treatment of adult patients with metastatic squamous NSCLC. (1.2)
- as a single agent for the first-line treatment of adult patients with NSCLC expressing PD-L1 [Tumor Proportion Score (TPS) ≥1%] as determined by an FDA-authorized test, with no EGFR or ALK genomic tumor aberrations, and is:
- as a single agent for the treatment of adult patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-authorized test, with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA QLEX. (1.2, 2.1)
- for the treatment of adult patients with resectable (tumors ≥4 cm or node positive) NSCLC in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery. (1.2)
- as a single agent, for adjuvant treatment following resection and platinum-based chemotherapy for adult patients with Stage IB (T2a ≥4 cm), II, or IIIA NSCLC. (1.2)
Malignant Pleural Mesothelioma (MPM)
- in combination with pemetrexed and platinum chemotherapy, as first-line treatment of adult patients with unresectable advanced or metastatic MPM. (1.3)
Head and Neck Squamous Cell Cancer (HNSCC)
- for the treatment of adult patients with resectable locally advanced HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-authorized test, as a single agent as neoadjuvant treatment, continued as adjuvant treatment in combination with radiotherapy (RT) with or without cisplatin and then as a single agent. (1.4)
- in combination with platinum and FU for the first-line treatment of adult patients with metastatic or with unresectable, recurrent HNSCC. (1.4)
- as a single agent for the first-line treatment of adult patients with metastatic or with unresectable, recurrent HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-authorized test. (1.4, 2.1)
- as a single agent for the treatment of adult patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy. (1.4)
Urothelial Cancer
- in combination with enfortumab vedotin, for the treatment of adult patients with locally advanced or metastatic urothelial cancer. (1.5)
- as a single agent for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma who:
- are not eligible for any platinum-containing chemotherapy, or
- who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. (1.5)
- in combination with enfortumab vedotin, as neoadjuvant treatment and then continued after cystectomy as adjuvant treatment of adult patients with muscle invasive bladder cancer (MIBC) who are ineligible for cisplatin-containing chemotherapy. (1.5)
- as a single agent for the treatment of adult patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. (1.5)
Microsatellite Instability-High or Mismatch Repair Deficient Cancer
- for the treatment of adult and pediatric patients 12 years and older with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors, as determined by an FDA-authorized test, that have progressed following prior treatment and who have no satisfactory alternative treatment options. (1.6, 2.1)
Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer (CRC)
- for the treatment of adult patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-authorized test. (1.7, 2.1)
Gastric Cancer
- in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test. (1.8)
- in combination with fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test. (1.8)
Esophageal Cancer
- for the treatment of adult patients with locally advanced or metastatic esophageal or gastroesophageal junction (GEJ) (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma that is not amenable to surgical resection or definitive chemoradiation either:
- in combination with platinum- and fluoropyrimidine-based chemotherapy for patients whose tumors express PD-L1 (CPS ≥1), or
- as a single agent after one or more prior lines of systemic therapy for patients with tumors of squamous cell histology that express PD-L1 (CPS ≥10) as determined by an FDA-authorized test. (1.9, 2.1)
Cervical Cancer
- in combination with chemoradiotherapy, for the treatment of adult patients with locally advanced cervical cancer involving the lower third of the vagina, with or without extension to pelvic sidewall, or hydronephrosis/non-functioning kidney, or spread to adjacent pelvic organs (FIGO 2014 Stage III-IVA). (1.10)
- in combination with chemotherapy, with or without bevacizumab, for the treatment of adult patients with persistent, recurrent, or metastatic cervical cancer whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test. (1.10, 2.1)
- as a single agent for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test. (1.10, 2.1)
Hepatocellular Carcinoma (HCC)
- for the treatment of adult patients with HCC secondary to hepatitis B who have received prior systemic therapy other than a PD-1/PD-L1-containing regimen. (1.11)
Biliary Tract Cancer (BTC)
- in combination with gemcitabine and cisplatin, for the treatment of adult patients with locally advanced unresectable or metastatic biliary tract cancer. (1.12)
Merkel Cell Carcinoma (MCC)
- for the treatment of adult and pediatric patients 12 years and older with recurrent locally advanced or metastatic Merkel cell carcinoma. (1.13)
Renal Cell Carcinoma (RCC)
- in combination with axitinib, for the first-line treatment of adult patients with advanced RCC. (1.14)
- in combination with lenvatinib, for the first-line treatment of adult patients with advanced RCC. (1.14)
- for the adjuvant treatment of adult patients with RCC at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions. (1.14)
Endometrial Carcinoma
- in combination with carboplatin and paclitaxel, followed by KEYTRUDA QLEX as a single agent, for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma. (1.15)
- in combination with lenvatinib, for the treatment of adult patients with advanced endometrial carcinoma that is mismatch repair proficient (pMMR) or not MSI-H as determined by an FDA-authorized test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation. (1.15, 2.1)
- as a single agent, for the treatment of adult patients with advanced endometrial carcinoma that is MSI-H or dMMR, as determined by an FDA-authorized test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation. (1.15, 2.1)
Tumor Mutational Burden-High (TMB-H) Cancer
- for the treatment of adult and pediatric patients 12 years and older with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-authorized test, that have progressed following prior treatment and who have no satisfactory alternative treatment options.1 (1.16, 2.1)
- Limitations of Use: The safety and effectiveness of KEYTRUDA QLEX in pediatric patients 12 years and older with TMB-H central nervous system cancers have not been established.
Cutaneous Squamous Cell Carcinoma (cSCC)
- for the treatment of adult patients with recurrent or metastatic cSCC or locally advanced cSCC that is not curable by surgery or radiation. (1.17)
Triple-Negative Breast Cancer (TNBC)
- for the treatment of adult patients with high-risk early-stage TNBC in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery. (1.18)
- in combination with chemotherapy, for the treatment of adult patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10) as determined by an FDA-authorized test. (1.18, 2.1)
Ovarian Cancer
- in combination with paclitaxel, with or without bevacizumab, is indicated for the treatment of adult patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal carcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test, and who have received one or two prior systemic treatment regimens. (1.19, 2.1)
1 This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
DOSAGE AND ADMINISTRATION
KEYTRUDA QLEX has different recommended dosage and administration than intravenous pembrolizumab. (2.2)
- KEYTRUDA QLEX is for subcutaneous use in the thigh or abdomen only. (2.2)
- Do not administer KEYTRUDA QLEX intravenously. (2.2)
- KEYTRUDA QLEX must be administered by a healthcare provider. (2.2)
The recommended dose for adults and pediatric patients 12 years and older who weigh greater than 40 kg is:
- Every 3-week dosing (395 mg/4,800 units): Inject 2.4 mL subcutaneously in the abdomen or thigh over 1 minute. (2.3)
- Every 6-week dosing (790 mg/9,600 units): Inject 4.8 mL subcutaneously in the abdomen or thigh over 2 minutes. (2.3)
- For RCC, administer KEYTRUDA QLEX as a single agent in the adjuvant setting, or in the advanced setting with either:
- axitinib 5 mg orally twice daily or
- lenvatinib 20 mg orally once daily. (2.3)
- For Endometrial Carcinoma, administer KEYTRUDA QLEX:
- in combination with carboplatin and paclitaxel regardless of MMR or MSI status, or
- in combination with lenvatinib 20 mg orally once daily for pMMR or not MSI-H tumors, or
- as a single agent for MSI-H or dMMR tumors. (2.3)
DOSAGE FORMS AND STRENGTHS
Injection: (3)
- 395 mg pembrolizumab and 4,800 units berahyaluronidase alfa per 2.4 mL (165 mg/2,000 units per mL) in a single-dose vial
- 790 mg pembrolizumab and 9,600 units berahyaluronidase alfa per 4.8 mL (165 mg/2,000 units per mL) in a single-dose vial
CONTRAINDICATIONS
KEYTRUDA QLEX is contraindicated in patients with known hypersensitivity to berahyaluronidase alfa, hyaluronidase or to any of its excipients. (4)
WARNINGS AND PRECAUTIONS
- Immune-Mediated Adverse Reactions (5.1)
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions, and solid organ transplant rejection.
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment.
- Withhold or permanently discontinue based on severity and type of reaction.
- Hypersensitivity and Administration-Related Reactions: Interrupt injection and resume upon symptom resolution, or permanently discontinue KEYTRUDA QLEX based on the severity of reaction. (5.2)
- Complications of Allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. (5.3)
- Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials. (5.4)
- Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective method of contraception. (5.5, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) in patients treated with KEYTRUDA QLEX in combination with chemotherapy were nausea, fatigue, and musculoskeletal pain. (6.1)
The safety of KEYTRUDA QLEX for the approved indications is also based on the safety of intravenous pembrolizumab given as a single agent or in combination with other antitumor medicines.
The most common adverse reactions (reported in ≥20% of patients) with intravenous pembrolizumab were:
- As a single agent: fatigue, musculoskeletal pain, rash, diarrhea, pyrexia, cough, decreased appetite, pruritus, dyspnea, constipation, pain, abdominal pain, nausea, and hypothyroidism. (6.1)
- In combination with chemotherapy or chemoradiotherapy: fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, dyspnea, pyrexia, alopecia, peripheral neuropathy, mucosal inflammation, stomatitis, headache, weight loss, abdominal pain, arthralgia, myalgia, insomnia, palmar-plantar erythrodysesthesia, urinary tract infection, hypothyroidism, radiation skin injury, dysphagia, dry mouth and musculoskeletal pain. (6.1)
- In combination with chemotherapy and bevacizumab: peripheral neuropathy, alopecia, anemia, fatigue/asthenia, nausea, neutropenia, diarrhea, hypertension, thrombocytopenia, constipation, arthralgia, vomiting, urinary tract infection, rash, leukopenia, hypothyroidism, decreased appetite, pyrexia, epistaxis, decreased white blood cell count, and stomatitis. (6.1)
- In combination with axitinib: diarrhea, fatigue/asthenia, hypertension, hepatotoxicity, hypothyroidism, decreased appetite, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation. (6.1)
- In combination with lenvatinib: hypothyroidism, hypertension, fatigue, diarrhea, musculoskeletal disorders, nausea, decreased appetite, vomiting, stomatitis, weight loss, abdominal pain, urinary tract infection, proteinuria, constipation, headache, hemorrhagic events, palmar-plantar erythrodysesthesia, dysphonia, rash, hepatotoxicity, and acute kidney injury. (6.1)
- In combination with enfortumab vedotin: rash, peripheral neuropathy, fatigue, pruritus, diarrhea, alopecia, weight loss, decreased appetite, dry eye, nausea, constipation, dysgeusia, and urinary tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Melanoma
1.2 Non-Small Cell Lung Cancer
1.3 Malignant Pleural Mesothelioma
1.4 Head and Neck Squamous Cell Cancer
1.5 Urothelial Cancer
1.6 Microsatellite Instability-High or Mismatch Repair Deficient Cancer
1.7 Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer
1.8 Gastric Cancer
1.9 Esophageal Cancer
1.10 Cervical Cancer
1.11 Hepatocellular Carcinoma
1.12 Biliary Tract Cancer
1.13 Merkel Cell Carcinoma
1.14 Renal Cell Carcinoma
1.15 Endometrial Carcinoma
1.16 Tumor Mutational Burden-High Cancer
1.17 Cutaneous Squamous Cell Carcinoma
1.18 Triple-Negative Breast Cancer
1.19 Ovarian Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Important Dosage and Administration Information
2.3 Recommended Dosage
2.4 Dosage Modifications
2.5 Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
5.2 Hypersensitivity and Administration-Related Reactions
5.3 Complications of Allogeneic HSCT
5.4 Increased Mortality in Patients with Multiple Myeloma when Pembrolizumab is Added to a Thalidomide Analogue and Dexamethasone
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 KEYTRUDA QLEX
14.2 Melanoma
14.3 Non-Small Cell Lung Cancer
14.4 Malignant Pleural Mesothelioma
14.5 Head and Neck Squamous Cell Cancer
14.6 Urothelial Cancer
14.7 Microsatellite Instability-High or Mismatch Repair Deficient Cancer
14.8 Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer
14.9 Gastric Cancer
14.10 Esophageal Cancer
14.11 Cervical Cancer
14.12 Hepatocellular Carcinoma
14.13 Biliary Tract Cancer
14.14 Merkel Cell Carcinoma
14.15 Renal Cell Carcinoma
14.16 Endometrial Carcinoma
14.17 Tumor Mutational Burden-High Cancer
14.18 Cutaneous Squamous Cell Carcinoma
14.19 Triple-Negative Breast Cancer
14.20 Ovarian Cancer
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Melanoma
KEYTRUDA QLEX™ is indicated for the treatment of adult patients with unresectable or metastatic melanoma.
KEYTRUDA QLEX is indicated for the adjuvant treatment of adult and pediatric patients 12 years and older with Stage IIB, IIC, or III melanoma following complete resection.
1.2 Non-Small Cell Lung Cancer
KEYTRUDA QLEX, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of adult patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
KEYTRUDA QLEX, in combination with carboplatin and either paclitaxel or paclitaxel protein-bound, is indicated for the first-line treatment of adult patients with metastatic squamous NSCLC.
KEYTRUDA QLEX, as a single agent, is indicated for the first-line treatment of adult patients with NSCLC expressing PD-L1 [Tumor Proportion Score (TPS) ≥1%] as determined by an FDA-authorized test [see Dosage and Administration (2.1)], with no EGFR or ALK genomic tumor aberrations, and is:
- Stage III where patients are not candidates for surgical resection or definitive chemoradiation, or
- metastatic.
KEYTRUDA QLEX, as a single agent, is indicated for the treatment of adult patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-authorized test [see Dosage and Administration (2.1)], with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA QLEX.
KEYTRUDA QLEX is indicated for the treatment of adult patients with resectable (tumors ≥4 cm or node positive) NSCLC in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
KEYTRUDA QLEX, as a single agent, is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with Stage IB (T2a ≥4 cm), II, or IIIA NSCLC.
1.3 Malignant Pleural Mesothelioma
KEYTRUDA QLEX, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of adult patients with unresectable advanced or metastatic malignant pleural mesothelioma (MPM).
1.4 Head and Neck Squamous Cell Cancer
KEYTRUDA QLEX is indicated for the treatment of adult patients with resectable locally advanced HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-authorized test [see Dosage and Administration (2.1)], as a single agent as neoadjuvant treatment, continued as adjuvant treatment in combination with radiotherapy (RT) with or without cisplatin and then as a single agent.
KEYTRUDA QLEX, in combination with platinum and fluorouracil (FU), is indicated for the first-line treatment of adult patients with metastatic or with unresectable, recurrent head and neck squamous cell carcinoma (HNSCC).
KEYTRUDA QLEX, as a single agent, is indicated for the first-line treatment of adult patients with metastatic or with unresectable, recurrent HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
KEYTRUDA QLEX, as a single agent, is indicated for the treatment of adult patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy.
1.5 Urothelial Cancer
KEYTRUDA QLEX, in combination with enfortumab vedotin, is indicated for the treatment of adult patients with locally advanced or metastatic urothelial cancer.
KEYTRUDA QLEX, as a single agent, is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma:
- who are not eligible for any platinum-containing chemotherapy, or
- who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
KEYTRUDA QLEX, in combination with enfortumab vedotin, as neoadjuvant treatment and then continued after cystectomy as adjuvant treatment, is indicated for the treatment of adult patients with muscle invasive bladder cancer (MIBC) who are ineligible for cisplatin-containing chemotherapy.
KEYTRUDA QLEX, as a single agent, is indicated for the treatment of adult patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
1.6 Microsatellite Instability-High or Mismatch Repair Deficient Cancer
KEYTRUDA QLEX is indicated for the treatment of adult and pediatric patients 12 years and older with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors, as determined by an FDA-authorized test, that have progressed following prior treatment and who have no satisfactory alternative treatment options [see Dosage and Administration (2.1)].
1.7 Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer
KEYTRUDA QLEX is indicated for the treatment of adult patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
1.8 Gastric Cancer
KEYTRUDA QLEX, in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
KEYTRUDA QLEX, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥ 1) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
1.9 Esophageal Cancer
KEYTRUDA QLEX is indicated for the treatment of adult patients with locally advanced or metastatic esophageal or gastroesophageal junction (GEJ) (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma that is not amenable to surgical resection or definitive chemoradiation either:
- in combination with platinum- and fluoropyrimidine-based chemotherapy for patients with tumors that express PD-L1 (CPS ≥ 1) [see Dosage and Administration (2.1)], or
- as a single agent after one or more prior lines of systemic therapy for patients with tumors of squamous cell histology that express PD-L1 (CPS ≥10) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
1.10 Cervical Cancer
KEYTRUDA QLEX, in combination with chemoradiotherapy (CRT), is indicated for the treatment of adult patients with locally advanced cervical cancer involving the lower third of the vagina, with or without extension to pelvic sidewall, or hydronephrosis/non-functioning kidney, or spread to adjacent pelvic organs (FIGO 2014 Stage III-IVA).
KEYTRUDA QLEX, in combination with chemotherapy, with or without bevacizumab, is indicated for the treatment of adult patients with persistent, recurrent, or metastatic cervical cancer whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
KEYTRUDA QLEX, as a single agent, is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
1.11 Hepatocellular Carcinoma
KEYTRUDA QLEX is indicated for the treatment of adult patients with hepatocellular carcinoma (HCC) secondary to hepatitis B who have received prior systemic therapy other than a PD-1/PD-L1-containing regimen.
1.12 Biliary Tract Cancer
KEYTRUDA QLEX, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced unresectable or metastatic biliary tract cancer (BTC).
1.13 Merkel Cell Carcinoma
KEYTRUDA QLEX is indicated for the treatment of adult and pediatric patients 12 years and older with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC).
1.14 Renal Cell Carcinoma
KEYTRUDA QLEX, in combination with axitinib, is indicated for the first-line treatment of adult patients with advanced renal cell carcinoma (RCC).
KEYTRUDA QLEX, in combination with lenvatinib, is indicated for the first-line treatment of adult patients with advanced RCC.
KEYTRUDA QLEX is indicated for the adjuvant treatment of adult patients with RCC at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions [see Clinical Studies (14.15)].
1.15 Endometrial Carcinoma
KEYTRUDA QLEX, in combination with carboplatin and paclitaxel, followed by KEYTRUDA QLEX as a single agent, is indicated for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma.
KEYTRUDA QLEX, in combination with lenvatinib, is indicated for the treatment of adult patients with advanced endometrial carcinoma that is mismatch repair proficient (pMMR) or not MSI-H as determined by an FDA-authorized test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation [see Dosage and Administration (2.1)].
KEYTRUDA QLEX, as a single agent, is indicated for the treatment of adult patients with advanced endometrial carcinoma that is MSI-H or dMMR, as determined by an FDA-authorized test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation [see Dosage and Administration (2.1)].
1.16 Tumor Mutational Burden-High Cancer
KEYTRUDA QLEX is indicated for the treatment of adult and pediatric patients 12 years and older with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-authorized test [see Dosage and Administration (2.1)], that have progressed following prior treatment and who have no satisfactory alternative treatment options.
This indication is approved under accelerated approval based on tumor response rate and durability of response [see Clinical Studies (14.17)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Limitations of Use: The safety and effectiveness of KEYTRUDA QLEX in pediatric patients 12 years and older with TMB-H central nervous system cancers have not been established.
1.17 Cutaneous Squamous Cell Carcinoma
KEYTRUDA QLEX is indicated for the treatment of adult patients with recurrent or metastatic cutaneous squamous cell carcinoma (cSCC) or locally advanced cSCC that is not curable by surgery or radiation.
1.18 Triple-Negative Breast Cancer
KEYTRUDA QLEX is indicated for the treatment of adult patients with high-risk early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
KEYTRUDA QLEX, in combination with chemotherapy, is indicated for the treatment of adult patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10) as determined by an FDA-authorized test [see Dosage and Administration (2.1)].
1.19 Ovarian Cancer
KEYTRUDA QLEX, in combination with paclitaxel, with or without bevacizumab, is indicated for the treatment of adult patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal carcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-authorized test [see Dosage and Administration (2.1)], and who have received one or two prior systemic treatment regimens.
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
See information on FDA-authorized tests for intravenous pembrolizumab. Information on FDA-authorized tests for patient selection is available at: http://www.fda.gov/CompanionDiagnostics.
Patient Selection for Single-Agent Treatment
Select patients for treatment with KEYTRUDA QLEX as a single agent based on the presence of positive PD-L1 expression in:
- Stage III NSCLC who are not candidates for surgical resection or definitive chemoradiation [see Clinical Studies (14.3)].
- metastatic NSCLC [see Clinical Studies (14.3)].
- first-line treatment of metastatic or unresectable, recurrent HNSCC [see Clinical Studies (14.5)].
- previously treated recurrent locally advanced or metastatic esophageal cancer [see Clinical Studies (14.10)].
- recurrent or metastatic cervical cancer with disease progression on or after chemotherapy [see Clinical Studies (14.11)].
For the MSI-H/dMMR indications, select patients for treatment with KEYTRUDA QLEX as a single agent based on MSI-H/dMMR status in tumor specimens [see Clinical Studies (14.7, 14.8)].
For the TMB-H indication, select patients for treatment with KEYTRUDA QLEX as a single agent based on TMB-H status in tumor specimens [see Clinical Studies (14.17)].
Because subclonal dMMR mutations and microsatellite instability may arise in high-grade gliomas during temozolomide therapy, it is recommended to test for TMB-H, MSI-H, and dMMR in the primary tumor specimens obtained prior to initiation of temozolomide chemotherapy in patients with high-grade gliomas.
Additional Patient Selection Information for MSI-H or dMMR in Patients with non-CRC Solid Tumors
Due to discordance between local tests and FDA-authorized tests, confirmation of MSI-H or dMMR status is recommended by an FDA-authorized test in patients with MSI-H or dMMR solid tumors, if feasible. If unable to perform confirmatory MSI-H/dMMR testing, the presence of TMB ≥10 mut/Mb, as determined by an FDA-authorized test, may be used to select patients for treatment [see Clinical Studies (14.7)].
Patient Selection for Combination Therapy
For use of KEYTRUDA QLEX as a single agent as neoadjuvant treatment, then in combination with radiotherapy (RT) with or without chemotherapy then continued as a single agent as adjuvant treatment, select patients based on presence of positive PD-L1 expression (CPS ≥1) in resectable locally advanced HNSCC [see Clinical Studies (14.5)].
For use of KEYTRUDA QLEX in combination with chemotherapy, select patients based on the presence of positive PD-L1 expression (CPS ≥1) in locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma, and esophageal or gastroesophageal junction (GEJ) carcinoma [see Clinical Studies (14.9), (14.10)].
- An FDA-authorized test for the detection of PD-L1 for the selection of patients with PD-L1 (CPS ≥1) expression in esophageal carcinoma in combination with platinum- and fluoropyrimidine-based chemotherapy is not available.
For use of KEYTRUDA QLEX in combination with chemotherapy, with or without bevacizumab, select patients based on the presence of positive PD-L1 expression in persistent, recurrent, or metastatic cervical cancer [see Clinical Studies (14.11)].
For the pMMR/not MSI-H advanced endometrial carcinoma indication, select patients for treatment with KEYTRUDA QLEX in combination with lenvatinib based on MMR or MSI status in tumor specimens [see Clinical Studies (14.16)].
For use of KEYTRUDA QLEX in combination with chemotherapy, select patients based on the presence of positive PD-L1 expression in locally recurrent unresectable or metastatic TNBC [see Clinical Studies (14.19)].
For use of KEYTRUDA QLEX in combination with paclitaxel, with or without bevacizumab, select patients based on the presence of positive PD-L1 expression (CPS ≥1) in platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal carcinoma [see Clinical Studies (14.20)].
2.2 Important Dosage and Administration Information
- KEYTRUDA QLEX has different recommended dosage and administration instructions than intravenous pembrolizumab.
- To reduce the risk of medication errors, check the vial labels to ensure that the drug being prepared and administered is KEYTRUDA QLEX for subcutaneous use and not intravenous pembrolizumab.
- Do not substitute KEYTRUDA QLEX with intravenous pembrolizumab because they have different recommended dosages and routes of administration.
- Patients receiving intravenous pembrolizumab can switch to subcutaneous KEYTRUDA QLEX at their next scheduled dose.
- Patients receiving subcutaneous KEYTRUDA QLEX can switch to intravenous pembrolizumab at their next scheduled dose.
- Administer KEYTRUDA QLEX as a subcutaneous injection into the thigh or abdomen, avoiding the 5 cm area around the navel.
- Every 3-week dosing (395 mg/4,800 units): inject 2.4 mL subcutaneously over 1 minute. Treatment duration is provided in Recommended Dosage (Table 1).
- Every 6-week dosing (790 mg/9,600 units): inject 4.8 mL subcutaneously over 2 minutes. Treatment duration is provided in Recommended Dosage (Table 1).
- Inject into healthy skin and never into areas where the skin is red, bruised, tender, or hard.
- Ensure the injection site is at least 2.5 cm from the previous injection site.
- During treatment with KEYTRUDA QLEX, do not administer other medications for subcutaneous use at the same site as KEYTRUDA QLEX.
- Do not administer KEYTRUDA QLEX intravenously.
- KEYTRUDA QLEX must be administered by a healthcare provider.
2.3 Recommended Dosage
The recommended dosages of KEYTRUDA QLEX are presented in Table 1.
- Every 3-week dosing (395 mg pembrolizumab and 4,800 units berahyaluronidase alfa): inject 2.4 mL subcutaneously over 1 minute.
- Every 6-week dosing (790 mg pembrolizumab and 9,600 units berahyaluronidase alfa): inject 4.8 mL subcutaneously over 2 minutes.
Table 1: Recommended Dosage Indication Recommended Dosage of
KEYTRUDA QLEXDuration/Timing of Treatment - * The recommended dosage for melanoma, MSI-H or dMMR cancer, MCC and TMB-H cancer has not been established in pediatric patients 12 years and older who weigh 40 kg or less [see Use in Specific Populations (8.4)].
- † Refer to the Prescribing Information for the agents administered in combination with KEYTRUDA QLEX for recommended dosing information, as appropriate.
- ‡ When axitinib is used in combination with KEYTRUDA QLEX, dose escalation of axitinib above the initial 5 mg dose may be considered at intervals of six weeks or longer.
- § Patients who experience disease progression or unacceptable toxicity related to KEYTRUDA QLEX with neoadjuvant treatment in combination with chemotherapy should not receive adjuvant single agent KEYTRUDA QLEX.
Monotherapy Adult patients with unresectable or
metastatic melanoma395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeksUntil disease progression or unacceptable
toxicityAdjuvant treatment of adult patients
with melanoma, NSCLC, or RCC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeksUntil disease recurrence, unacceptable
toxicity, or up to 12 monthsAdult patients with NSCLC, HNSCC,
locally advanced or metastatic
Urothelial Carcinoma, MSI-H or dMMR
Cancer, MSI-H or dMMR CRC, MSI-H
or dMMR Endometrial Carcinoma,
Esophageal Cancer, Cervical Cancer,
HCC, MCC, TMB-H Cancer, or cSCC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeksUntil disease progression, unacceptable
toxicity, or up to 24 monthsAdult patients with high-risk BCG-
unresponsive NMIBC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeksUntil persistent or recurrent high-risk
NMIBC, disease progression,
unacceptable toxicity, or up to 24 monthsPediatric patients* (12 years and older
who weigh greater than 40 kg) with
MSI-H or dMMR Cancer, MCC, or TMB-
H Cancer395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeksUntil disease progression, unacceptable
toxicity, or up to 24 monthsPediatric patients* (12 years and older
who weigh greater than 40 kg) for
adjuvant treatment of melanoma
395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeksUntil disease recurrence, unacceptable
toxicity, or up to 12 monthsCombination Therapy† Adult patients with resectable NSCLC 395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior
to chemotherapy when given on
the same day.Neoadjuvant treatment in combination with
chemotherapy for 12 weeks or until
disease progression that precludes
definitive surgery or unacceptable toxicity,
followed by adjuvant treatment with
KEYTRUDA QLEX as a single agent after
surgery for 39 weeks or until disease
recurrence or unacceptable toxicityAdult patients with NSCLC, MPM,
HNSCC, HER2-negative Gastric
Cancer, Esophageal Cancer, or BTC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior
to chemotherapy when given on
the same day.Until disease progression, unacceptable
toxicity, or up to 24 monthsAdult patients with locally advanced or
metastatic urothelial cancer395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX after
enfortumab vedotin when given
on the same day.Until disease progression, unacceptable
toxicity, or up to 24 monthsAdult patients with locally advanced
HNSCC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to
cisplatin when given on the same day.Neoadjuvant: - Administer KEYTRUDA QLEX for 6 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity.
- Administer KEYTRUDA QLEX in combination with RT with or without cisplatin.
- Continue KEYTRUDA QLEX as a single agent.
disease recurrence or unacceptable
toxicity or up to one yearAdult patients with MIBC 395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX after
enfortumab vedotin when given on the
same day.Neoadjuvant: - Administer KEYTRUDA QLEX 395 mg/4,800 units every 3 weeks for 3 doses in combination with enfortumab vedotin or until disease progression that precludes curative-intent cystectomy or unacceptable toxicity.
- Administer KEYTRUDA QLEX 395 mg/4,800 units every 3 weeks for 14 doses or 790 mg/9,600 units every 6 weeks for 7 doses in combination with enfortumab vedotin or until disease recurrence or unacceptable toxicity
Adult patients with HER2-positive
Gastric Cancer395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to
trastuzumab and chemotherapy
when given on the same day.Until disease progression, unacceptable
toxicity, or up to 24 monthsAdult patients with Cervical Cancer 395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to
chemoradiotherapy or prior to
chemotherapy with or without
bevacizumab when given on the
same day.Until disease progression, unacceptable
toxicity, or for KEYTRUDA QLEX, up to
24 monthsAdult patients with RCC 395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX in
combination with axitinib 5 mg
orally twice daily‡
or
Administer KEYTRUDA QLEX in combination with lenvatinib 20 mg orally once daily.Until disease progression, unacceptable
toxicity, or for KEYTRUDA QLEX, up to
24 monthsAdult patients with Endometrial
Carcinoma395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to
carboplatin and paclitaxel when
given on the same day.
or
Administer KEYTRUDA QLEX in
combination with lenvatinib
20 mg orally once daily.Until disease progression, unacceptable
toxicity, or for KEYTRUDA QLEX, up to
24 monthsAdult patients with high-risk early-stage
TNBC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to chemotherapy when given on the same day.Neoadjuvant treatment in combination with chemotherapy for 24 weeks (8 doses of 395 mg/4,800 units every 3 weeks or 4 doses of 790 mg/9,600 units every 6 weeks) or until disease progression or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA QLEX as a single agent for up to 27 weeks (9 doses of 395 mg/4,800 units every 3 weeks or 5 doses of 790 mg/9,600 units every 6 weeks) or until disease recurrence or unacceptable toxicity§ Adult patients with locally recurrent
unresectable or metastatic TNBC395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to
chemotherapy when given on
the same day.Until disease progression, unacceptable
toxicity, or up to 24 monthsAdult patients with Ovarian Cancer 395 mg/4,800 units every 3 weeks
or
790 mg/9,600 units every 6 weeks
Administer KEYTRUDA QLEX prior to
paclitaxel with or without bevacizumab
when given on the same day.Until disease progression,
unacceptable toxicity, or up to
24 months2.4 Dosage Modifications
No dose reduction for KEYTRUDA QLEX is recommended. In general, withhold KEYTRUDA QLEX for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue KEYTRUDA QLEX for Life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating steroids.
Dosage modifications for KEYTRUDA QLEX for adverse reactions that require management different from these general guidelines are summarized in Table 2.
Table 2: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity* Dosage Modification ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson Syndrome, TEN = toxic epidermal necrolysis, ULN = upper limit normal - * Based on Common Terminology Criteria for Adverse Events (CTCAE), version 4.0
- † Resume in patients with complete or partial resolution (Grades 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating steroids or inability to reduce prednisone to 10 mg per day or less (or equivalent) within 12 weeks of initiating steroids.
- ‡ If AST and ALT are less than or equal to ULN at baseline, withhold or permanently discontinue KEYTRUDA QLEX based on recommendations for hepatitis with no liver involvement.
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] Pneumonitis Grade 2 Withhold† Grade 3 or 4 Permanently discontinue Colitis Grade 2 or 3 Withhold† Grade 4 Permanently discontinue
Hepatitis with no tumor involvement
of the liverAST or ALT increases to more than 3
and up to 8 times ULN
or
Total bilirubin increases to more than
1.5 and up to 3 times ULNWithhold† For liver enzyme elevations in
patients treated with combination
therapy with axitinib, see Table 3.AST or ALT increases to more than
8 times ULN
or
Total bilirubin increases to more than
3 times ULNPermanently discontinue Hepatitis with tumor involvement of
the liver‡Baseline AST or ALT is more than 1
and up to 3 times ULN and increases to
more than 5 and up to 10 times ULN
or
Baseline AST or ALT is more than 3
and up to 5 times ULN and increases to
more than 8 and up to 10 times ULNWithhold† ALT or AST increases to more than
10 times ULN
or
Total bilirubin increases to more than
3 times ULNPermanently discontinue Endocrinopathies Grade 3 or 4 Withhold until clinically stable or permanently
discontinue depending on severityNephritis with Renal Dysfunction Grade 2 or 3 increased blood creatinine Withhold† Grade 4 increased blood creatinine Permanently discontinue Exfoliative Dermatologic Conditions Suspected SJS, TEN, or DRESS Withhold† Confirmed SJS, TEN, or DRESS Permanently discontinue Myocarditis Grade 2, 3, or 4 Permanently discontinue Neurological Toxicities Grade 2 Withhold† Grade 3 or 4 Permanently discontinue Other Adverse Reactions Hypersensitivity and Administration-Related Systemic Reactions
[see Warnings and Precautions (5.2)]Grade 1 or 2 Interrupt injection (if not already fully administered). If symptoms resolve, resume injection Grade 3 or 4 Permanently discontinue The following table represents dosage modifications that are different from those described above for KEYTRUDA QLEX or in the Full Prescribing Information for the drug administered in combination.
Table 3: Recommended Specific Dosage Modifications for Adverse Reactions for KEYTRUDA QLEX in Combination with Axitinib Treatment Adverse Reaction Severity Dosage Modification ALT = alanine aminotransferase, AST = aspartate aminotransferase, ULN = upper limit normal - * Consider corticosteroid therapy
- † Based on Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Consider rechallenge with a single drug or sequential rechallenge with both drugs after recovery. If rechallenging with axitinib, consider dose reduction as per the axitinib Prescribing Information.
KEYTRUDA QLEX in
combination with
axitinibLiver enzyme elevations* ALT or AST increases to at least 3 times but less than 10 times ULN without concurrent total bilirubin at least 2 times ULN Withhold both KEYTRUDA QLEX
and axitinib until resolution to
Grades 0 or 1†ALT or AST increases to more than 3 times ULN with concurrent total bilirubin at least 2 times ULN
or ALT or AST ≥10 times ULNPermanently discontinue both
KEYTRUDA QLEX and axitinibRecommended Dose Modifications for Adverse Reactions for KEYTRUDA QLEX in Combination with Lenvatinib
When administering KEYTRUDA QLEX in combination with lenvatinib, modify the dosage of one or both drugs. Withhold or discontinue KEYTRUDA QLEX as shown in Table 2. Refer to lenvatinib prescribing information for additional dose modification information.
2.5 Preparation
KEYTRUDA QLEX is a ready-to-use solution. Do not dilute KEYTRUDA QLEX.
Do not shake.
Preparation of the Syringe
- Remove KEYTRUDA QLEX vial from refrigerated storage [2°C to 8°C (36°F to 46°F)] and allow it to equilibrate to room temperature [20°C to 25°C (68°F to 77°F)] for at least 30 minutes.
- Prior to preparation for administration, if needed, the unpunctured vial may be stored at room temperature for up to 24 hours.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution is clear to slightly opalescent, colorless to slightly yellow. Discard the vial if visible particles are observed.
- Use a sterile, polypropylene or polycarbonate syringe and a stainless steel transfer needle (18 to 21 gauge) to withdraw KEYTRUDA QLEX from the vial.
- Every 3-week dosing (395 mg pembrolizumab/4,800 units berahyaluronidase alfa): withdraw 2.4 mL into the syringe.
- Every 6-week dosing (790 mg pembrolizumab/9,600 units berahyaluronidase alfa): withdraw 4.8 mL into the syringe.
- To avoid needle clogging, change the needle to a 25 to 30 gauge, ½-inch, stainless steel hypodermic injection needle immediately prior to subcutaneous injection.
- Discard any unused portion left in the vial.
Storage of Prepared Syringe
The product does not contain a preservative and should be used immediately after withdrawing from the vial. If not used immediately, store the syringe containing KEYTRUDA QLEX with the transfer needle and cap in place:
- At room temperature 20°C to 25°C (68°F to 77°F) for up to 8 hours, or
- In the refrigerator at 2°C to 8°C (36°F to 46°F) for up to 24 hours. The 24-hour period may include up to 8 hours at room temperature.
Discard if storage time exceeds these limits.
If refrigerated, allow the filled syringe to come to room temperature for at least 30 minutes prior to administration.
Do not freeze.
-
3 DOSAGE FORMS AND STRENGTHS
KEYTRUDA QLEX is a clear to slightly opalescent, colorless to slightly yellow solution provided as:
- Injection: 395 mg pembrolizumab and 4,800 units berahyaluronidase alfa per 2.4 mL (165 mg/2,000 units per mL) in a single-dose vial
- Injection: 790 mg pembrolizumab and 9,600 units berahyaluronidase alfa per 4.8 mL (165 mg/2,000 units per mL) in a single-dose vial
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
KEYTRUDA QLEX is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death-receptor 1 (PD-1) or the PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under WARNINGS AND PRECAUTIONS may not include all possible severe and fatal immune-mediated adverse reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. For patients with TNBC treated with KEYTRUDA QLEX in the neoadjuvant setting, monitor blood cortisol at baseline, prior to surgery, and as clinically indicated. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue KEYTRUDA QLEX depending on severity [see Dosage and Administration (2.4)]. In general, if KEYTRUDA QLEX requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
KEYTRUDA QLEX can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Immune-mediated pneumonitis occurred in 5% (13/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including fatal (0.4%), Grade 3 (2%), and Grade 2 (1.2%) adverse reactions.
Intravenous Pembrolizumab as a Single Agent
Immune-mediated pneumonitis occurred in 3.4% (94/2799) of patients receiving intravenous pembrolizumab, including fatal (0.1%), Grade 4 (0.3%), Grade 3 (0.9%), and Grade 2 (1.3%) adverse reactions. Systemic corticosteroids were required in 67% (63/94) of patients with pneumonitis. Pneumonitis led to permanent discontinuation of intravenous pembrolizumab in 1.3% (36) of patients and withholding of intravenous pembrolizumab in 0.9% (26) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement; of these, 23% had recurrence of pneumonitis. Pneumonitis resolved in 59% of the 94 patients.
In a clinical study enrolling 580 adult patients with resected NSCLC (KEYNOTE-091) who received intravenous pembrolizumab as a single agent for adjuvant treatment, pneumonitis occurred in 41 (7%) patients, including fatal (0.2%), Grade 4 (0.3%), and Grade 3 (1%) adverse reactions. Patients received high-dose corticosteroids for a median duration of 10 days (range: 1 day to 2.3 months). Pneumonitis led to discontinuation of intravenous pembrolizumab in 26 (4.5%) of patients. Of the patients who developed pneumonitis, 54% interrupted intravenous pembrolizumab, 63% discontinued intravenous pembrolizumab, and 71% had resolution.
Immune-Mediated Colitis
KEYTRUDA QLEX can cause immune-mediated colitis, which may present with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. Immune-mediated colitis occurred in 1.2% (3/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 3 (0.8%), and Grade 2 (0.4%) adverse reactions.
Intravenous Pembrolizumab as a Single Agent
Immune-mediated colitis occurred in 1.7% (48/2799) of patients receiving intravenous pembrolizumab, including Grade 4 (<0.1%), Grade 3 (1.1%), and Grade 2 (0.4%) adverse reactions. Systemic corticosteroids were required in 69% (33/48) of patients with colitis. Additional immunosuppressant therapy was required in 4.2% of patients. Colitis led to permanent discontinuation of intravenous pembrolizumab in 0.5% (15) of patients and withholding of intravenous pembrolizumab in 0.5% (13) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement; of these, 23% had recurrence of colitis. Colitis resolved in 85% of the 48 patients.
Hepatotoxicity and Immune-Mediated Hepatitis
KEYTRUDA QLEX can cause immune-mediated hepatitis. Immune-mediated hepatitis occurred in 0.4% (1/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 2 (0.4%) adverse reactions.
Intravenous Pembrolizumab as a Single Agent
Immune-mediated hepatitis occurred in 0.7% (19/2799) of patients receiving intravenous pembrolizumab, including Grade 4 (<0.1%), Grade 3 (0.4%), and Grade 2 (0.1%) adverse reactions. Systemic corticosteroids were required in 68% (13/19) of patients with hepatitis. Eleven percent of these patients required additional immunosuppressant therapy. Hepatitis led to permanent discontinuation of intravenous pembrolizumab in 0.2% (6) of patients and withholding of intravenous pembrolizumab in 0.3% (9) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement; of these, none had recurrence of hepatitis. Hepatitis resolved in 79% of the 19 patients.
In Combination with Axitinib
KEYTRUDA QLEX in combination with axitinib can cause hepatic toxicity with higher than expected frequencies of Grades 3 and 4 ALT and AST elevations compared to KEYTRUDA QLEX alone. Monitor liver enzymes before initiation of and periodically throughout treatment. Consider more frequent monitoring of liver enzymes as compared to when the drugs are administered as single agents. For elevated liver enzymes, interrupt KEYTRUDA QLEX and axitinib, and consider administering corticosteroids as needed [see Dosage and Administration (2.4)].
Intravenous Pembrolizumab in Combination with Axitinib
With the combination of intravenous pembrolizumab and axitinib, Grades 3 and 4 increased ALT (20%) and increased AST (13%) were seen. Fifty-nine percent of the patients with increased ALT received systemic corticosteroids. In patients with ALT ≥3 times ULN (Grades 2-4, n=116), ALT resolved to Grades 0-1 in 94%. Among the 92 patients who were rechallenged with either intravenous pembrolizumab (n=3) or axitinib (n=34) administered as a single agent or with both (n=55), recurrence of ALT ≥3 times ULN was observed in 1 patient receiving intravenous pembrolizumab, 16 patients receiving axitinib, and 24 patients receiving both intravenous pembrolizumab and axitinib. All patients with a recurrence of ALT ≥3 ULN subsequently recovered from the event.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
KEYTRUDA QLEX can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold KEYTRUDA QLEX depending on severity [see Dosage and Administration (2.4)].
Adrenal insufficiency occurred in 2% (5/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 3 (0.4%), and Grade 2 (0.8%) adverse reactions.
Intravenous Pembrolizumab as a Single Agent
Adrenal insufficiency occurred in 0.8% (22/2799) of patients receiving intravenous pembrolizumab, including Grade 4 (<0.1%), Grade 3 (0.3%), and Grade 2 (0.3%) adverse reactions. Systemic corticosteroids were required in 77% (17/22) of patients with adrenal insufficiency; of these, the majority remained on systemic corticosteroids. Adrenal insufficiency led to permanent discontinuation of intravenous pembrolizumab in <0.1% (1) of patients and withholding of intravenous pembrolizumab in 0.3% (8) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement.
Hypophysitis
KEYTRUDA QLEX can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as indicated. Withhold or permanently discontinue KEYTRUDA QLEX depending on severity [see Dosage and Administration (2.4)].
Intravenous Pembrolizumab as a Single Agent
Hypophysitis occurred in 0.6% (17/2799) of patients receiving intravenous pembrolizumab, including Grade 4 (<0.1%), Grade 3 (0.3%), and Grade 2 (0.2%) adverse reactions. Systemic corticosteroids were required in 94% (16/17) of patients with hypophysitis; of these, the majority remained on systemic corticosteroids. Hypophysitis led to permanent discontinuation of intravenous pembrolizumab in 0.1% (4) of patients and withholding of intravenous pembrolizumab in 0.3% (7) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement.
Thyroid Disorders
KEYTRUDA QLEX can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue KEYTRUDA QLEX depending on severity [see Dosage and Administration (2.4)].
Thyroiditis occurred in 0.4% (1/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 2 (0.4%). Hyperthyroidism occurred in 8% (20/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 2 (3.2%). Hypothyroidism occurred in 14% (35/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 2 (11%).
Intravenous Pembrolizumab as a Single Agent
Thyroiditis occurred in 0.6% (16/2799) of patients receiving intravenous pembrolizumab, including Grade 2 (0.3%). No patients discontinued intravenous pembrolizumab due to thyroiditis. Intravenous pembrolizumab was withheld in <0.1% (1) of patients.
Hyperthyroidism occurred in 3.4% (96/2799) of patients receiving intravenous pembrolizumab, including Grade 3 (0.1%) and Grade 2 (0.8%). Hyperthyroidism led to permanent discontinuation of intravenous pembrolizumab in <0.1% (2) of patients and withholding of intravenous pembrolizumab in 0.3% (7) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement.
The incidence of new or worsening hyperthyroidism was higher in 580 patients with resected NSCLC, occurring in 11% of patients receiving intravenous pembrolizumab as a single agent as adjuvant treatment (KEYNOTE-091), including Grade 3 (0.2%) hyperthyroidism.
Hypothyroidism occurred in 8% (237/2799) of patients receiving intravenous pembrolizumab, including Grade 3 (0.1%) and Grade 2 (6.2%). Hypothyroidism led to permanent discontinuation of intravenous pembrolizumab in <0.1% (1) of patients and withholding of intravenous pembrolizumab in 0.5% (14) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement. The majority of patients with hypothyroidism required long-term thyroid hormone replacement.
The incidence of new or worsening hypothyroidism was higher in 1185 patients with HNSCC, occurring in 16% of patients receiving intravenous pembrolizumab as a single agent or in combination with platinum and FU, including Grade 3 (0.3%) hypothyroidism.
The incidence of new or worsening hypothyroidism was higher in 580 patients with resected NSCLC, occurring in 22% of patients receiving intravenous pembrolizumab as a single agent as adjuvant treatment (KEYNOTE-091), including Grade 3 (0.3%) hypothyroidism.
Type 1 Diabetes Mellitus, which can present with Diabetic Ketoacidosis
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold KEYTRUDA QLEX depending on severity [see Dosage and Administration (2.4)].
Type 1 diabetes mellitus occurred in 0.4% (1/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy.
Intravenous Pembrolizumab as a Single Agent
Type 1 diabetes mellitus occurred in 0.2% (6/2799) of patients receiving intravenous pembrolizumab. Type 1 diabetes mellitus led to permanent discontinuation in <0.1% (1) of patients and withholding of intravenous pembrolizumab in <0.1% (1) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement. All patients with Type 1 diabetes mellitus required long-term insulin therapy.
Immune-Mediated Nephritis with Renal Dysfunction
KEYTRUDA QLEX can cause immune-mediated nephritis.
Intravenous Pembrolizumab as a Single Agent
Immune-mediated nephritis occurred in 0.3% (9/2799) of patients receiving intravenous pembrolizumab, including Grade 4 (<0.1%), Grade 3 (0.1%), and Grade 2 (0.1%) adverse reactions. Systemic corticosteroids were required in 89% (8/9) of patients with nephritis. Nephritis led to permanent discontinuation of intravenous pembrolizumab in 0.1% (3) of patients and withholding of intravenous pembrolizumab in 0.1% (3) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement; of these, none had recurrence of nephritis. Nephritis resolved in 56% of the 9 patients.
Immune-Mediated Dermatologic Adverse Reactions
KEYTRUDA QLEX can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens Johnson Syndrome, DRESS, and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue KEYTRUDA QLEX depending on severity [see Dosage and Administration (2.4)].
Immune-mediated dermatologic adverse reactions occurred in 1.6% (4/251) of patients receiving KEYTRUDA QLEX in combination with chemotherapy, including Grade 4 (0.8%), and Grade 3 (0.8%) adverse reactions.
Intravenous Pembrolizumab as a Single Agent
Immune-mediated dermatologic adverse reactions occurred in 1.4% (38/2799) of patients receiving intravenous pembrolizumab, including Grade 3 (1%) and Grade 2 (0.1%) adverse reactions. Systemic corticosteroids were required in 40% (15/38) of patients with immune-mediated dermatologic adverse reactions. Immune-mediated dermatologic adverse reactions led to permanent discontinuation of intravenous pembrolizumab in 0.1% (2) of patients and withholding of intravenous pembrolizumab in 0.6% (16) of patients. All patients who were withheld reinitiated intravenous pembrolizumab after symptom improvement; of these, 6% had recurrence of immune-mediated dermatologic adverse reactions. Immune-mediated dermatologic adverse reactions resolved in 79% of the 38 patients.
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received KEYTRUDA QLEX, intravenous pembrolizumab, or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions.
Cardiac/Vascular: Myocarditis, pericarditis, vasculitis
Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy
Ocular: Uveitis, iritis and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis, to include increases in serum amylase and lipase levels, gastritis (2.8%), duodenitis
Musculoskeletal and Connective Tissue: Myositis/polymyositis, rhabdomyolysis (and associated sequelae, including renal failure), arthritis (1.5%), polymyalgia rheumatica
Endocrine: Hypoparathyroidism
Hematologic/Immune: Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection, other transplant (including corneal graft) rejection
5.2 Hypersensitivity and Administration-Related Reactions
KEYTRUDA QLEX can cause severe or life-threatening administration-related reactions, including hypersensitivity and anaphylaxis. In Study MK-3475A-D77, hypersensitivity and administration-related systemic reactions occurred in 3.2% (8/251) of patients receiving KEYTRUDA QLEX, including Grade 2 (2.8%). Monitor patients for signs and symptoms of administration-related systemic reactions including rigors, chills, wheezing, pruritus, flushing, rash, hypotension, hypoxemia, and fever. Interrupt injection (if not already fully administered) and resume if symptoms resolve for mild or moderate hypersensitivity and administration-related systemic reactions. For severe or life-threatening hypersensitivity and administration-related systemic reactions, stop injection and permanently discontinue KEYTRUDA QLEX [see Dosage and Administration (2.4)].
5.3 Complications of Allogeneic HSCT
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
5.4 Increased Mortality in Patients with Multiple Myeloma when Pembrolizumab is Added to a Thalidomide Analogue and Dexamethasone
In two randomized trials in patients with multiple myeloma, the addition of intravenous pembrolizumab to a thalidomide analogue plus dexamethasone, a use for which no PD-1 or PD-L1 blocking antibody is indicated, resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled trials.
5.5 Embryo-Fetal Toxicity
Based on its mechanism of action, KEYTRUDA QLEX can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-L1/PD-1 pathway can lead to increased risk of immune-related rejection of the developing fetus resulting in fetal death. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with KEYTRUDA QLEX and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Severe and fatal immune-mediated adverse reactions [see Warnings and Precautions (5.1)].
- Hypersensitivity and Administration-Related Reactions [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS reflect exposure to intravenous pembrolizumab as a single agent in 2799 patients in three randomized, open-label, active-controlled trials (KEYNOTE-002, KEYNOTE-006, and KEYNOTE-010), which enrolled 912 patients with melanoma and 682 patients with NSCLC, and one single-arm trial (KEYNOTE-001), which enrolled 655 patients with melanoma and 550 patients with NSCLC. In addition to the 2799 patients, certain subsections in the WARNINGS AND PRECAUTIONS describe adverse reactions observed with exposure to KEYTRUDA QLEX in combination with platinum doublet chemotherapy in a randomized, open-label, active-controlled trial (Study MK-3475A-D77), which enrolled 251 patients with NSCLC; intravenous pembrolizumab as a single agent in a randomized, placebo-controlled trial (KEYNOTE-091), which enrolled 580 patients with resected NSCLC; a non-randomized, open-label, multi-cohort trial (KEYNOTE-012), a non-randomized, open-label, single-cohort trial (KEYNOTE-055), and two randomized, open-label, active-controlled trials (KEYNOTE-040 and KEYNOTE-048 single agent arms), which enrolled 909 patients with HNSCC; in a randomized, open-label, active-controlled trial (KEYNOTE-048 combination arm), which enrolled 276 patients with HNSCC; in combination with axitinib in a randomized, active-controlled trial (KEYNOTE-426), which enrolled 429 patients with RCC; and in post-marketing use. Across all trials, patients were administered either KEYTRUDA QLEX 790 mg/9,600 units every 6 weeks or intravenous pembrolizumab at doses of 2 mg/kg every 3 weeks, 10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks, or 200 mg every 3 weeks. Among the 2799 patients who received intravenous pembrolizumab, 41% were exposed for 6 months or more and 21% were exposed for 12 months or more.
The most common adverse reactions (≥20%) in patients who received KEYTRUDA QLEX in combination with chemotherapy were nausea (25%), fatigue (25%), and musculoskeletal pain (21%).
The safety of KEYTRUDA QLEX for the approved indications is also based on the safety of intravenous pembrolizumab given as a single agent or in combination with other antitumor medicines.
The most common adverse reactions (≥20%) in patients who received intravenous pembrolizumab were:
- as a single agent: fatigue, musculoskeletal pain, rash, diarrhea, pyrexia, cough, decreased appetite, pruritus, dyspnea, constipation, pain, abdominal pain, nausea, and hypothyroidism.
- in combination with chemotherapy or chemoradiotherapy: fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, dyspnea, pyrexia, alopecia, peripheral neuropathy, mucosal inflammation, stomatitis, headache, weight loss, abdominal pain, arthralgia, myalgia, insomnia, palmar-plantar erythrodysesthesia, urinary tract infection, hypothyroidism, radiation skin injury, dysphagia, dry mouth, and musculoskeletal pain.
- in combination with chemotherapy and bevacizumab: peripheral neuropathy, alopecia, anemia, fatigue/asthenia, nausea, neutropenia, diarrhea, hypertension, thrombocytopenia, constipation, arthralgia, vomiting, urinary tract infection, rash, leukopenia, hypothyroidism, decreased appetite, pyrexia, epistaxis, decreased white blood cell count, and stomatitis.
- in combination with axitinib: diarrhea, fatigue/asthenia, hypertension, hepatotoxicity, hypothyroidism, decreased appetite, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation.
- in combination with lenvatinib: hypothyroidism, hypertension, fatigue, diarrhea, musculoskeletal disorders, nausea, decreased appetite, vomiting, stomatitis, weight loss, abdominal pain, urinary tract infection, proteinuria, constipation, headache, hemorrhagic events, palmar-plantar erythrodysesthesia, dysphonia, rash, hepatotoxicity, and acute kidney injury.
- in combination with enfortumab vedotin: rash, peripheral neuropathy, fatigue, pruritus, diarrhea, alopecia, weight loss, decreased appetite, dry eye, nausea, constipation, dysgeusia, and urinary tract infection.
Adverse Reactions in Patients with NSCLC Treated with KEYTRUDA QLEX
The safety of KEYTRUDA QLEX compared to intravenous pembrolizumab in patients with previously untreated, metastatic NSCLC with no EGFR, ALK or ROS1 genomic tumor aberrations was evaluated in Study MK-3475A-D77 [see Clinical Studies (14.1)]. A total of 377 patients received either KEYTRUDA QLEX 790 mg/9,600 units every 6 weeks in combination with platinum doublet chemotherapy (n=251) or intravenous pembrolizumab 400 mg every 6 weeks in combination with platinum doublet chemotherapy (n=126).
Among patients who received KEYTRUDA QLEX, 58% were exposed for 6 months or longer and 3.2% were exposed for greater than one year.
The median age of patients who received KEYTRUDA QLEX was 65 years (range: 39 to 87); 73% male, 63% White; 29% Asian, 4.8% multiple races, 2% Black or African American, 0.8% Alaska Native or American Indian; and 29% were of Hispanic or Latino ethnicity.
Serious adverse reactions occurred in 39% of patients who received KEYTRUDA QLEX in combination with chemotherapy. Serious adverse reactions in ≥1% of patients who received KEYTRUDA QLEX were pneumonia (10%), thrombocytopenia (4%), febrile neutropenia (4%), neutropenia (2.8%), musculoskeletal pain (2%), pneumonitis (2%), diarrhea (1.6%), rash (1.2%), respiratory failure (1.2%), and anemia (1.2%). Fatal adverse reactions occurred in 10% of patients who received KEYTRUDA QLEX in combination with chemotherapy including pneumonia (3.2%), neutropenic sepsis (2%), death not otherwise specified (1.6%), respiratory failure (1.2%), parotitis (0.4%), pneumonitis (0.4%), pneumothorax (0.4%), pulmonary embolism (0.4%), neutropenic colitis (0.4%), and seizure (0.4%).
Permanent discontinuation of KEYTRUDA QLEX due to an adverse reaction occurred in 16% of patients. Adverse reactions which resulted in permanent discontinuation of KEYTRUDA QLEX in ≥2% of patients included pneumonia and pneumonitis.
Dosage interruptions of KEYTRUDA QLEX due to an adverse reaction occurred in 45% of patients. Adverse reactions which required dosage interruption in ≥2% of patients included neutropenia, anemia, thrombocytopenia, pneumonia, rash, and increased aspartate aminotransferase.
Tables 4 and 5 summarize the adverse reactions and laboratory abnormalities, respectively, in patients on KEYTRUDA QLEX in Study MK-3475A-D77.
Table 4: Adverse Reactions Occurring in ≥10% of Patients with Metastatic NSCLC Receiving KEYTRUDA QLEX in Study MK-3475A-D77 Adverse Reaction KEYTRUDA QLEX and
Platinum Doublet ChemotherapyIntravenous Pembrolizumab and
Platinum Doublet Chemotherapy(n=251) (n=126) All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE V5.0
- † Includes diarrhea, colitis, and enterocolitis.
- ‡ Includes fatigue, asthenia.
- § Includes musculoskeletal pain, arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal stiffness, myalgia, non-cardiac chest pain, and pain in extremity.
- ¶ Includes rash, dermatitis, dermatitis acneiform, dermatitis bullous, dermatitis exfoliative, eczema, erythema multiforme, immune-mediated dermatitis, rash erythematous, rash follicular, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and skin exfoliation.
- # Includes pneumonia, COVID-19 pneumonia, lower respiratory tract infection, lung abscess, pneumocystis jirovecii pneumonia, pneumonia bacterial, and pneumonia mycoplasmal.
- Þ Includes neuropathy peripheral, hypoaesthesia, neuralgia, paraesthesia, and peripheral sensory neuropathy.
- ß Includes cough, productive cough, and upper-airway cough syndrome.
Gastrointestinal Nausea 25 1.2 25 0.8 Diarrhea† 16 2 14 0.8 Constipation 14 0 18 1.6 General Fatigue‡ 25 3.6 26 3.2 Musculoskeletal and Connective Tissue Musculoskeletal pain§ 21 2.4 30 2.4 Skin and Subcutaneous Tissue Rash¶ 18 2 19 0.8 Pruritus 12 0 13 0.8 Endocrine Hypothyroidism 14 0 12 0 Infections Pneumonia# 17 10 16 7 Nervous System Peripheral neuropathyÞ 11 0.4 14 0 Metabolism and Nutrition Decreased appetite 11 0.8 21 2.4 Hyperglycemia 11 0.8 11 0.8 Respiratory, Thoracic and Mediastinal Coughß 10 0 11 0.8 Clinically relevant adverse reactions in <10% of patients who received KEYTRUDA QLEX included local injection site reactions (2.4%).
Table 5: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients with Metastatic NSCLC Receiving KEYTRUDA QLEX in Study MK-3475A-D77 Laboratory Test* KEYTRUDA QLEX and
Platinum Doublet ChemotherapyIntravenous Pembrolizumab and
Platinum Doublet ChemotherapyAll Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: KEYTRUDA QLEX plus platinum doublet chemotherapy (range: 240 to 246 patients) and intravenous pembrolizumab plus platinum doublet chemotherapy (range: 124 to 125 patients).
- † Graded per NCI CTCAE V5.0
Hematology Anemia 80 22 86 26 Leukopenia 61 13 52 10 Neutropenia 58 28 49 19 Lymphopenia 55 22 54 18 Thrombocytopenia 43 11 41 6 Chemistry Increased AST 43 2.5 38 3.2 Hypoalbuminemia 38 0.4 39 0 Increased ALT 37 2.1 36 0.8 Hyponatremia 35 4.1 42 7 Increased creatinine 33 4.5 38 6 Hypocalcemia 31 2.1 31 2.4 Increased alkaline phosphatase 29 0.4 34 0 Hypokalemia 21 5 24 6 Adverse Reactions in Adult and Pediatric Patients Treated with Intravenous Pembrolizumab
The safety of KEYTRUDA QLEX for its approved indications [see Indications and Usage (1)] has been established in adequate and well-controlled studies of KEYTRUDA QLEX in combination with platinum doublet chemotherapy (Study MK-3475A-D77) and intravenous pembrolizumab, as a single agent or in combination therapy, across tumor types.
Below is a description of adverse reactions of intravenous pembrolizumab in these adequate and well-controlled studies.
Melanoma
Ipilimumab-Naive Melanoma
The safety of intravenous pembrolizumab for the treatment of patients with unresectable or metastatic melanoma who had not received prior ipilimumab and who had received no more than one prior systemic therapy was investigated in KEYNOTE-006. KEYNOTE-006 was a multicenter, open-label, active-controlled trial where patients were randomized (1:1:1) and received intravenous pembrolizumab 10 mg/kg every 2 weeks (n=278) or intravenous pembrolizumab 10 mg/kg every 3 weeks (n=277) until disease progression or unacceptable toxicity or ipilimumab 3 mg/kg every 3 weeks for 4 doses unless discontinued earlier for disease progression or unacceptable toxicity (n=256) [see Clinical Studies (14.2)]. Patients with autoimmune disease, a medical condition that required systemic corticosteroids or other immunosuppressive medication; a history of interstitial lung disease; or active infection requiring therapy, including HIV or hepatitis B or C, were ineligible.
The median duration of exposure was 5.6 months (range: 1 day to 11.0 months) for intravenous pembrolizumab and similar in both treatment arms. Fifty-one and 46% of patients received intravenous pembrolizumab 10 mg/kg every 2 or 3 weeks, respectively, for ≥6 months. No patients in either arm received treatment for more than one year.
The study population characteristics were: median age of 62 years (range: 18 to 89); 60% male; 98% White; 32% had an elevated lactate dehydrogenase (LDH) value at baseline; 65% had M1c stage disease; 9% with history of brain metastasis; and approximately 36% had been previously treated with systemic therapy which included a BRAF inhibitor (15%), chemotherapy (13%), and immunotherapy (6%).
In KEYNOTE-006, the adverse reaction profile was similar for the every 2 week and every 3 week schedule, therefore summary safety results are provided in a pooled analysis (n=555) of both intravenous pembrolizumab arms. Adverse reactions leading to permanent discontinuation of intravenous pembrolizumab occurred in 9% of patients. Adverse reactions leading to discontinuation of intravenous pembrolizumab in more than one patient were colitis (1.4%), autoimmune hepatitis (0.7%), allergic reaction (0.4%), polyneuropathy (0.4%), and cardiac failure (0.4%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 21% of patients; the most common (≥1%) was diarrhea (2.5%). Tables 6 and 7 summarize selected adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-006.
Table 6: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-006 Adverse Reaction Intravenous Pembrolizumab
10 mg/kg every 2 or 3 weeksIpilimumab n=555 n=256 All Grades†
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in the ipilimumab arm
- † Graded per NCI CTCAE v4.0
- ‡ Includes rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and exfoliative rash.
- § Includes skin hypopigmentation
General Fatigue 28 0.9 28 3.1 Skin and Subcutaneous Tissue Rash‡ 24 0.2 23 1.2 Vitiligo§ 13 0 2 0 Musculoskeletal and Connective Tissue Arthralgia 18 0.4 10 1.2 Back pain 12 0.9 7 0.8 Respiratory, Thoracic and Mediastinal Cough 17 0 7 0.4 Dyspnea 11 0.9 7 0.8 Metabolism and Nutrition Decreased appetite 16 0.5 14 0.8 Nervous System Headache 14 0.2 14 0.8 Other clinically important adverse reactions occurring in ≥10% of patients receiving intravenous pembrolizumab were diarrhea (26%), nausea (21%), and pruritus (17%).
Table 7: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Melanoma Patients Receiving Intravenous Pembrolizumab in KEYNOTE-006 Laboratory Test† Intravenous Pembrolizumab
10 mg/kg every 2 or 3 weeksIpilimumab All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than in ipilimumab arm
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (520 to 546 patients) and ipilimumab (237 to 247 patients); hypertriglyceridemia: intravenous pembrolizumab n=429 and ipilimumab n=183; hypercholesterolemia: intravenous pembrolizumab n=484 and ipilimumab n=205.
- ‡ Graded per NCI CTCAE v4.0
Chemistry Hyperglycemia 45 4.2 45 3.8 Hypertriglyceridemia 43 2.6 31 1.1 Hyponatremia 28 4.6 26 7 Increased AST 27 2.6 25 2.5 Hypercholesterolemia 20 1.2 13 0 Hematology Anemia 35 3.8 33 4.0 Lymphopenia 33 7 25 6 Other laboratory abnormalities occurring in ≥20% of patients receiving intravenous pembrolizumab were increased hypoalbuminemia (27% all Grades; 2.4% Grades 3-4), increased ALT (23% all Grades; 3.1% Grades 3-4), and increased alkaline phosphatase (21% all Grades, 2% Grades 3-4).
Ipilimumab-Refractory Melanoma
The safety of intravenous pembrolizumab in patients with unresectable or metastatic melanoma with disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor, was investigated in KEYNOTE-002. KEYNOTE-002 was a multicenter, partially blinded (intravenous pembrolizumab dose), randomized (1:1:1), active-controlled trial in which 528 patients received intravenous pembrolizumab 2 mg/kg (n=178) or 10 mg/kg (n=179) every 3 weeks or investigator's choice of chemotherapy (n=171), consisting of dacarbazine (26%), temozolomide (25%), paclitaxel and carboplatin (25%), paclitaxel (16%), or carboplatin (8%) [see Clinical Studies (14.2)]. Patients with autoimmune disease, severe immune-related toxicity related to ipilimumab, defined as any Grade 4 toxicity or Grade 3 toxicity requiring corticosteroid treatment (greater than 10 mg/day prednisone or equivalent dose) for greater than 12 weeks; medical conditions that required systemic corticosteroids or other immunosuppressive medication; a history of interstitial lung disease; or an active infection requiring therapy, including HIV or hepatitis B or C, were ineligible.
The median duration of exposure to intravenous pembrolizumab 2 mg/kg every 3 weeks was 3.7 months (range: 1 day to 16.6 months) and to intravenous pembrolizumab 10 mg/kg every 3 weeks was 4.8 months (range: 1 day to 16.8 months). In the intravenous pembrolizumab 2 mg/kg arm, 36% of patients were exposed to intravenous pembrolizumab for ≥6 months and 4% were exposed for ≥12 months. In the 10 mg/kg arm, 41% of patients were exposed to intravenous pembrolizumab for ≥6 months and 6% of patients were exposed to intravenous pembrolizumab for ≥12 months.
The study population characteristics were: median age of 62 years (range: 15 to 89); 61% male; 98% White; 41% had an elevated LDH value at baseline; 83% had M1c stage disease; 73% received two or more prior therapies for advanced or metastatic disease (100% received ipilimumab and 25% a BRAF inhibitor); and 15% with history of brain metastasis.
In KEYNOTE-002, the adverse reaction profile was similar for the 2 mg/kg dose and 10 mg/kg dose, therefore summary safety results are provided in a pooled analysis (n=357) of both intravenous pembrolizumab arms. Adverse reactions resulting in permanent discontinuation occurred in 12% of patients receiving intravenous pembrolizumab; the most common (≥1%) were general physical health deterioration (1%), asthenia (1%), dyspnea (1%), pneumonitis (1%), and generalized edema (1%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 14% of patients; the most common (≥1%) were dyspnea (1%), diarrhea (1%), and maculo-papular rash (1%). Tables 8 and 9 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-002.
Table 8: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-002 Adverse Reaction Intravenous Pembrolizumab
2 mg/kg or 10 mg/kg every 3 weeksChemotherapy† n=357 n=171 All Grades‡
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in chemotherapy arm
- † Chemotherapy: dacarbazine, temozolomide, carboplatin plus paclitaxel, paclitaxel, or carboplatin
- ‡ Graded per NCI CTCAE v4.0
- § Includes rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, and rash pruritic
Skin and Subcutaneous Tissue Pruritus 28 0 8 0 Rash§ 24 0.6 8 0 Gastrointestinal Constipation 22 0.3 20 2.3 Diarrhea 20 0.8 20 2.3 Abdominal pain 13 1.7 8 1.2 Respiratory, Thoracic and Mediastinal Cough 18 0 16 0 General Pyrexia 14 0.3 9 0.6 Asthenia 10 2.0 9 1.8 Musculoskeletal and Connective Tissue Arthralgia 14 0.6 10 1.2 Other clinically important adverse reactions occurring in patients receiving intravenous pembrolizumab were fatigue (43%), nausea (22%), decreased appetite (20%), vomiting (13%), and peripheral neuropathy (1.7%).
Table 9: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Melanoma Patients Receiving Intravenous Pembrolizumab in KEYNOTE-002 Laboratory Test† Intravenous Pembrolizumab
2 mg/kg or 10 mg/kg every 3 weeksChemotherapy All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than in chemotherapy arm.
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 320 to 325 patients) and chemotherapy (range: 154 to 161 patients); hypertriglyceridemia: intravenous pembrolizumab n=247 and chemotherapy n=116; decreased bicarbonate: intravenous pembrolizumab n=263 and chemotherapy n=123.
- ‡ Graded per NCI CTCAE v4.0
Chemistry Hyperglycemia 49 6 44 6 Hypoalbuminemia 37 1.9 33 0.6 Hyponatremia 37 7 24 3.8 Hypertriglyceridemia 33 0 32 0.9 Increased alkaline phosphatase 26 3.1 18 1.9 Increased AST 24 2.2 16 0.6 Decreased bicarbonate 22 0.4 13 0 Hypocalcemia 21 0.3 18 1.9 Increased ALT 21 1.8 16 0.6 Other laboratory abnormalities occurring in ≥20% of patients receiving intravenous pembrolizumab were anemia (44% all Grades; 10% Grades 3-4) and lymphopenia (40% all Grades; 9% Grades 3-4).
Adjuvant Treatment of Resected Stage IIB or IIC Melanoma
Among the 969 patients with Stage IIB or IIC melanoma enrolled in KEYNOTE-716 [see Clinical Studies (14.2)] treated with intravenous pembrolizumab, the median duration of exposure to intravenous pembrolizumab was 9.9 months (range: 0 to 15.4 months). Patients with autoimmune disease or a medical condition that required immunosuppression or mucosal or ocular melanoma were ineligible. Adverse reactions occurring in patients with Stage IIB or IIC melanoma were similar to those occurring in 1011 patients with Stage III melanoma from KEYNOTE-054 or the 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent.
Adjuvant Treatment of Stage III Resected Melanoma
The safety of intravenous pembrolizumab as a single agent was investigated in KEYNOTE-054, a randomized (1:1) double-blind trial in which 1019 patients with completely resected Stage IIIA (>1 mm lymph node metastasis), IIIB or IIIC melanoma received 200 mg of intravenous pembrolizumab by intravenous infusion every 3 weeks (n=509) or placebo (n=502) for up to one year [see Clinical Studies (14.2)]. Patients with active autoimmune disease or a medical condition that required immunosuppression or mucosal or ocular melanoma were ineligible. Seventy-six percent of patients received intravenous pembrolizumab for 6 months or longer.
The study population characteristics were: median age of 54 years (range: 19 to 88), 25% age 65 or older; 62% male; and 94% ECOG PS of 0 and 6% ECOG PS of 1. Sixteen percent had Stage IIIA, 46% had Stage IIIB, 18% had Stage IIIC (1-3 positive lymph nodes), and 20% had Stage IIIC (≥4 positive lymph nodes).
Two patients treated with intravenous pembrolizumab died from causes other than disease progression; causes of death were drug reaction with eosinophilia and systemic symptoms and autoimmune myositis with respiratory failure. Serious adverse reactions occurred in 25% of patients receiving intravenous pembrolizumab. Adverse reactions leading to permanent discontinuation occurred in 14% of patients receiving intravenous pembrolizumab; the most common (≥1%) were pneumonitis (1.4%), colitis (1.2%), and diarrhea (1%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 19% of patients; the most common (≥1%) were diarrhea (2.4%), pneumonitis (2%), increased ALT (1.4%), arthralgia (1.4%), increased AST (1.4%), dyspnea (1%), and fatigue (1%). Tables 10 and 11 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-054.
Table 10: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-054 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
n=509Placebo
n=502All Grades†
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in placebo arm
- † Graded per NCI CTCAE v4.03
Gastrointestinal Diarrhea 28 1.2 26 1.2 Nausea 17 0.2 15 0 Skin and Subcutaneous Tissue Pruritus 19 0 12 0 Rash 13 0.2 9 0 Musculoskeletal and Connective Tissue Arthralgia 16 1.2 14 0 Endocrine Hypothyroidism 15 0 2.8 0 Hyperthyroidism 10 0.2 1.2 0 Respiratory, Thoracic and Mediastinal Cough 14 0 11 0 General Asthenia 11 0.2 8 0 Influenza like illness 11 0 8 0 Investigations Weight loss 11 0 8 0 Table 11: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Melanoma Patients Receiving Intravenous Pembrolizumab in KEYNOTE-054 Laboratory Test† Intravenous Pembrolizumab
200 mg every 3 weeksPlacebo All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than placebo.
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 502 to 505 patients) and placebo (range: 491 to 497 patients).
- ‡ Graded per NCI CTCAE v4.03
Chemistry Increased ALT 25 2.4 15 0.2 Increased AST 22 1.8 14 0.4 Hematology Lymphopenia 22 1 15 1.2 NSCLC
First-line treatment of metastatic nonsquamous NSCLC with pemetrexed and platinum chemotherapy
The safety of intravenous pembrolizumab in combination with pemetrexed and investigator's choice of platinum (either carboplatin or cisplatin) was investigated in KEYNOTE-189, a multicenter, double-blind, randomized (2:1), active-controlled trial in patients with previously untreated, metastatic nonsquamous NSCLC with no EGFR or ALK genomic tumor aberrations [see Clinical Studies (14.3)]. A total of 607 patients received intravenous pembrolizumab 200 mg, pemetrexed and platinum every 3 weeks for 4 cycles followed by intravenous pembrolizumab and pemetrexed (n=405) or placebo, pemetrexed, and platinum every 3 weeks for 4 cycles followed by placebo and pemetrexed (n=202). Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to intravenous pembrolizumab 200 mg every 3 weeks was 7.2 months (range: 1 day to 20.1 months). Sixty percent of patients in the intravenous pembrolizumab arm were exposed to intravenous pembrolizumab for ≥6 months. Seventy-two percent of patients received carboplatin.
The study population characteristics were: median age of 64 years (range: 34 to 84), 49% age 65 or older; 59% male; 94% White and 3% Asian; and 18% with history of brain metastases at baseline.
Intravenous pembrolizumab was discontinued for adverse reactions in 20% of patients. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab were pneumonitis (3%) and acute kidney injury (2%). Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 53% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (13%), asthenia/fatigue (7%), anemia (7%), thrombocytopenia (5%), diarrhea (4%), pneumonia (4%), increased blood creatinine (3%), dyspnea (2%), febrile neutropenia (2%), upper respiratory tract infection (2%), increased ALT (2%), and pyrexia (2%). Tables 12 and 13 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-189.
Table 12: Adverse Reactions Occurring in ≥20% of Patients in KEYNOTE-189 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
Pemetrexed
Platinum Chemotherapy
n=405Placebo
Pemetrexed
Platinum Chemotherapy
n=202All Grades*
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes asthenia and fatigue
- ‡ Includes genital rash, rash, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular.
Gastrointestinal Nausea 56 3.5 52 3.5 Constipation 35 1.0 32 0.5 Diarrhea 31 5 21 3.0 Vomiting 24 3.7 23 3.0 General Fatigue† 56 12 58 6 Pyrexia 20 0.2 15 0 Metabolism and Nutrition Decreased appetite 28 1.5 30 0.5 Skin and Subcutaneous Tissue Rash‡ 25 2.0 17 2.5 Respiratory, Thoracic and Mediastinal Cough 21 0 28 0 Dyspnea 21 3.7 26 5 Table 13: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients in KEYNOTE-189 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
Pemetrexed
Platinum ChemotherapyPlacebo
Pemetrexed
Platinum ChemotherapyAll Grades†
%Grades 3-4
%All Grades
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab /pemetrexed/platinum chemotherapy (range: 381 to 401 patients) and placebo/pemetrexed/platinum chemotherapy (range: 184 to 197 patients).
- † Graded per NCI CTCAE v4.03
Hematology Anemia 85 17 81 18 Lymphopenia 65 22 64 25 Neutropenia 50 21 41 19 Thrombocytopenia 30 12 29 8 Chemistry Hyperglycemia 63 9 60 7 Increased ALT 47 3.8 42 2.6 Increased AST 47 2.8 40 1.0 Hypoalbuminemia 39 2.8 39 1.1 Increased creatinine 37 4.2 25 1.0 Hyponatremia 32 7 23 6 Hypophosphatemia 30 10 28 14 Increased alkaline phosphatase 26 1.8 29 2.1 Hypocalcemia 24 2.8 17 0.5 Hyperkalemia 24 2.8 19 3.1 Hypokalemia 21 5 20 5 First-line treatment of metastatic squamous NSCLC with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy
The safety of intravenous pembrolizumab in combination with carboplatin and investigator's choice of either paclitaxel or paclitaxel protein-bound was investigated in KEYNOTE-407, a multicenter, double-blind, randomized (1:1), placebo-controlled trial in 558 patients with previously untreated, metastatic squamous NSCLC [see Clinical Studies (14.3)]. Safety data are available for the first 203 patients who received intravenous pembrolizumab and chemotherapy (n=101) or placebo and chemotherapy (n=102). Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to intravenous pembrolizumab was 7 months (range: 1 day to 12 months). Sixty-one percent of patients in the intravenous pembrolizumab arm were exposed to intravenous pembrolizumab for ≥6 months. A total of 139 of 203 patients (68%) received paclitaxel and 64 patients (32%) received paclitaxel protein-bound in combination with carboplatin.
The study population characteristics were: median age of 65 years (range: 40 to 83), 52% age 65 or older; 78% male; 83% White; and 9% with history of brain metastases.
Intravenous pembrolizumab was discontinued for adverse reactions in 15% of patients, with no single type of adverse reaction accounting for the majority. Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 43% of patients; the most common (≥2%) were thrombocytopenia (20%), neutropenia (11%), anemia (6%), asthenia (2%), and diarrhea (2%). The most frequent (≥2%) serious adverse reactions were febrile neutropenia (6%), pneumonia (6%), and urinary tract infection (3%).
The adverse reactions observed in KEYNOTE-407 were similar to those observed in KEYNOTE-189 with the exception that increased incidences of alopecia (47% vs. 36%) and peripheral neuropathy (31% vs. 25%) were observed in the intravenous pembrolizumab and chemotherapy arm compared to the placebo and chemotherapy arm in KEYNOTE-407.
Previously Untreated NSCLC
The safety of intravenous pembrolizumab was investigated in KEYNOTE-042, a multicenter, open-label, randomized (1:1), active-controlled trial in 1251 patients with PD-L1 expressing, previously untreated Stage III NSCLC who were not candidates for surgical resection or definitive chemoradiation or metastatic NSCLC [see Clinical Studies (14.3)]. Patients received intravenous pembrolizumab 200 mg every 3 weeks (n=636) or investigator's choice of chemotherapy (n=615), consisting of pemetrexed and carboplatin followed by optional pemetrexed (n=312) or paclitaxel and carboplatin followed by optional pemetrexed (n=303) every 3 weeks. Patients with EGFR or ALK genomic tumor aberrations; autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to intravenous pembrolizumab was 5.6 months (range: 1 day to 27.3 months). Forty-eight percent of patients in the intravenous pembrolizumab arm were exposed to intravenous pembrolizumab 200 mg for ≥6 months.
The study population characteristics were: median age of 63 years (range: 25 to 90), 45% age 65 or older; 71% male; and 64% White, 30% Asian, and 2% Black. Nineteen percent were Hispanic or Latino. Eighty-seven percent had metastatic disease (Stage IV), 13% had Stage III disease (2% Stage IIIA and 11% Stage IIIB), and 5% had treated brain metastases at baseline.
Intravenous pembrolizumab was discontinued for adverse reactions in 19% of patients. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab were pneumonitis (3.0%), death due to unknown cause (1.6%), and pneumonia (1.4%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 33% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were pneumonitis (3.1%), pneumonia (3.0%), hypothyroidism (2.2%), and increased ALT (2.0%). The most frequent (≥2%) serious adverse reactions were pneumonia (7%), pneumonitis (3.9%), pulmonary embolism (2.4%), and pleural effusion (2.2%).
Tables 14 and 15 summarize the adverse reactions and laboratory abnormalities, respectively, in patients treated with intravenous pembrolizumab in KEYNOTE-042.
Table 14: Adverse Reactions Occurring in ≥10% of Patients in KEYNOTE-042 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
n=636Chemotherapy
n=615All Grades*
(%)Grades 3-5
(%)All Grades
(%)Grades 3-5
(%)- * Graded per NCI CTCAE v4.03
- † Includes fatigue and asthenia
- ‡ Includes rash, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular.
General Fatigue† 25 3.1 33 3.9 Pyrexia 10 0.3 8 0 Metabolism and Nutrition Decreased appetite 17 1.7 21 1.5 Respiratory, Thoracic and Mediastinal Dyspnea 17 2.0 11 0.8 Cough 16 0.2 11 0.3 Skin and Subcutaneous Tissue Rash‡ 15 1.3 8 0.2 Gastrointestinal Constipation 12 0 21 0.2 Diarrhea 12 0.8 12 0.5 Nausea 12 0.5 32 1.1 Endocrine Hypothyroidism 12 0.2 1.5 0 Infections Pneumonia 12 7 9 6 Investigations Weight loss 10 0.9 7 0.2 Table 15: Laboratory Abnormalities Worsened from Baseline in ≥20% of Patients in KEYNOTE-042 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeksChemotherapy All Grades†
%Grades 3-4
%All Grades
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 598 to 610 patients) and chemotherapy (range: 585 to 598 patients); increased prothrombin INR: intravenous pembrolizumab n=203 and chemotherapy n=173.
- † Graded per NCI CTCAE v4.03
Chemistry Hyperglycemia 52 4.7 51 5 Increased ALT 33 4.8 34 2.9 Hypoalbuminemia 33 2.2 29 1.0 Increased AST 31 3.6 32 1.7 Hyponatremia 31 9 32 8 Increased alkaline phosphatase 29 2.3 29 0.3 Hypocalcemia 25 2.5 19 0.7 Hyperkalemia 23 3.0 20 2.2 Increased prothrombin INR 21 2.0 15 2.9 Hypophosphatemia 20 4.7 17 4.3 Hematology Anemia 43 4.4 79 19 Lymphopenia 30 7 42 13 Previously Treated NSCLC
The safety of intravenous pembrolizumab was investigated in KEYNOTE-010, a multicenter, open-label, randomized (1:1:1), active-controlled trial, in patients with advanced NSCLC who had documented disease progression following treatment with platinum-based chemotherapy and, if positive for EGFR or ALK genetic aberrations, appropriate therapy for these aberrations [see Clinical Studies (14.3)]. A total of 991 patients received intravenous pembrolizumab 2 mg/kg (n=339) or 10 mg/kg (n=343) every 3 weeks or docetaxel (n=309) at 75 mg/m2 every 3 weeks. Patients with autoimmune disease, medical conditions that required systemic corticosteroids or other immunosuppressive medication, or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible.
The median duration of exposure to intravenous pembrolizumab 2 mg/kg every 3 weeks was 3.5 months (range: 1 day to 22.4 months) and to intravenous pembrolizumab 10 mg/kg every 3 weeks was 3.5 months (range 1 day to 20.8 months). The data described below reflect exposure to intravenous pembrolizumab 2 mg/kg in 31% of patients exposed to intravenous pembrolizumab for ≥6 months. In the intravenous pembrolizumab 10 mg/kg arm, 34% of patients were exposed to intravenous pembrolizumab for ≥6 months.
The study population characteristics were: median age of 63 years (range: 20 to 88), 42% age 65 or older; 61% male; 72% White and 21% Asian; and 8% with advanced localized disease, 91% with metastatic disease, and 15% with history of brain metastases. Twenty-nine percent received two or more prior systemic treatments for advanced or metastatic disease.
In KEYNOTE-010, the adverse reaction profile was similar for the 2 mg/kg and 10 mg/kg dose, therefore summary safety results are provided in a pooled analysis (n=682). Treatment was discontinued for adverse reactions in 8% of patients receiving intravenous pembrolizumab. The most common adverse events resulting in permanent discontinuation of intravenous pembrolizumab was pneumonitis (1.8%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 23% of patients; the most common (≥1%) were diarrhea (1%), fatigue (1.3%), pneumonia (1%), liver enzyme elevation (1.2%), decreased appetite (1.3%), and pneumonitis (1%). Tables 16 and 17 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-010.
Table 16: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-010 Adverse Reaction Intravenous Pembrolizumab
2 or 10 mg/kg every 3 weeks
n=682Docetaxel
75 mg/m2 every 3 weeks
n=309All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in docetaxel arm
- † Graded per NCI CTCAE v4.0
- ‡ Includes rash, rash erythematous, rash macular, rash maculo-papular, rash papular, and rash pruritic
Metabolism and Nutrition Decreased appetite 25 1.5 23 2.6 Respiratory, Thoracic and Mediastinal Dyspnea 23 3.7 20 2.6 Cough 19 0.6 14 0 Gastrointestinal Nausea 20 1.3 18 0.6 Constipation 15 0.6 12 0.6 Vomiting 13 0.9 10 0.6 Skin and Subcutaneous Tissue Rash‡ 17 0.4 8 0 Pruritus 11 0 3 0.3 Musculoskeletal and Connective Tissue Arthralgia 11 1.0 9 0.3 Back pain 11 1.5 8 0.3 Other clinically important adverse reactions occurring in patients receiving intravenous pembrolizumab were fatigue (25%), diarrhea (14%), asthenia (11%) and pyrexia (11%).
Table 17: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of NSCLC Patients Receiving Intravenous Pembrolizumab in KEYNOTE-010 Laboratory Test† Intravenous Pembrolizumab
2 or 10 mg/kg every 3 weeksDocetaxel
75 mg/m2 every 3 weeksAll Grades‡
%Grades 3-4
%All Grades‡
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than in docetaxel arm.
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 631 to 638 patients) and docetaxel (range: 271 to 277 patients).
- ‡ Graded per NCI CTCAE v4.0
Chemistry Hyponatremia 32 8 27 2.9 Increased alkaline phosphatase 28 3.0 16 0.7 Increased AST 26 1.6 12 0.7 Increased ALT 22 2.7 9 0.4 Hypocalcemia 20 0.9 20 1.8 Other laboratory abnormalities occurring in ≥20% of patients receiving intravenous pembrolizumab were hyperglycemia (44% all Grades; 4.1% Grades 3-4), anemia (37% all Grades; 3.8% Grades 3-4), hypertriglyceridemia (36% all Grades; 1.8% Grades 3-4), lymphopenia (32% all Grades; 9% Grades 3-4), hypoalbuminemia (34% all Grades; 1.6% Grades 3-4), and hypercholesterolemia (20% all Grades; 0.7% Grades 3-4).
Neoadjuvant and Adjuvant Treatment of Resectable NSCLC
The safety of intravenous pembrolizumab in combination with neoadjuvant platinum-containing chemotherapy followed by surgery and continued adjuvant treatment with intravenous pembrolizumab as a single agent after surgery was investigated in KEYNOTE-671, a multicenter, randomized (1:1), double-blind, placebo-controlled trial in patients with previously untreated and resectable Stage II, IIIA, or IIIB (N2) NSCLC by AJCC 8th edition [see Clinical Studies (14.3)]. Patients with active autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible.
The median duration of exposure to intravenous pembrolizumab 200 mg every 3 weeks was 10.9 months (range: 1 day to 18.6 months). The study population characteristics were: median age of 64 years (range: 26 to 83), 45% age 65 or older, 7% age 75 or older; 71% male; 61% White, 31% Asian, 2% Black, 4% race not reported; 9% Hispanic or Latino.
Adverse reactions occurring in patients with resectable NSCLC receiving intravenous pembrolizumab in combination with platinum containing chemotherapy, given as neoadjuvant treatment and continued as single agent adjuvant treatment, were generally similar to those occurring in patients in other clinical trials across tumor types receiving intravenous pembrolizumab in combination with chemotherapy.
Neoadjuvant Phase of KEYNOTE-671
A total of 396 patients received at least 1 dose of intravenous pembrolizumab in combination with platinum-containing chemotherapy as neoadjuvant treatment and 399 patients received at least 1 dose of placebo in combination with platinum-containing chemotherapy as neoadjuvant treatment.
Serious adverse reactions occurred in 34% of patients who received intravenous pembrolizumab in combination with platinum-containing chemotherapy as neoadjuvant treatment; the most frequent (≥2%) serious adverse reactions were pneumonia (4.8%), venous thromboembolism (3.3%), and anemia (2%). Fatal adverse reactions occurred in 1.3% of patients, including death due to unknown cause (0.8%), sepsis (0.3%), and immune-mediated lung disease (0.3%).
Permanent discontinuation of any study drug due to an adverse reaction occurred in 18% of patients who received intravenous pembrolizumab in combination with platinum-containing chemotherapy as neoadjuvant treatment; the most frequent (≥1%) adverse reactions that led to permanent discontinuation of any study drug were acute kidney injury (1.8%), interstitial lung disease (1.8%), anemia (1.5%), neutropenia (1.5%), and pneumonia (1.3%).
Of the 396 intravenous pembrolizumab-treated patients and 399 placebo-treated patients who received neoadjuvant treatment, 6% (n=25) and 4.3% (n=17), respectively, did not receive surgery due to adverse reactions. The most frequent (≥1%) adverse reactions that led to cancellation of surgery in the intravenous pembrolizumab arm was interstitial lung disease (1%).
Of the 325 intravenous pembrolizumab-treated patients who received surgery, 3.1% (n=10) experienced delay of surgery (surgery more than 8 weeks from last neoadjuvant treatment if patient received less than 4 cycles of neoadjuvant therapy or more than 20 weeks after first dose of neoadjuvant treatment if patient received 4 cycles of neoadjuvant therapy) due to adverse reactions. Of the 317 placebo-treated patients who received surgery, 2.5% (n=8) experienced delay of surgery due to adverse reactions.
Of the 325 intravenous pembrolizumab-treated patients who received surgery, 7% (n=22) did not receive adjuvant treatment due to adverse reactions. Of the 317 placebo-treated patients who received surgery, 3.2% (n=10) did not receive adjuvant treatment due to adverse reactions.
Adjuvant Phase of KEYNOTE-671
A total of 290 patients in the intravenous pembrolizumab arm and 267 patients in the placebo arm received at least 1 dose of adjuvant treatment.
Of the patients who received single agent intravenous pembrolizumab as adjuvant treatment, 14% experienced serious adverse reactions; the most frequent serious adverse reaction was pneumonia (3.4%). One fatal adverse reaction of pulmonary hemorrhage occurred. Permanent discontinuation of adjuvant intravenous pembrolizumab due to an adverse reaction occurred in 12% of patients; the most frequent (≥1%) adverse reactions that led to permanent discontinuation of adjuvant intravenous pembrolizumab were diarrhea (1.7%), interstitial lung disease (1.4%), AST increased (1%), and musculoskeletal pain (1%).
Adjuvant Treatment of Resected NSCLC
The safety of intravenous pembrolizumab as a single agent was investigated in KEYNOTE-091, a multicenter, randomized (1:1), triple-blind, placebo-controlled trial in patients with completely resected Stage IB (T2a ≥4 cm), II, or IIIA NSCLC; adjuvant chemotherapy up to 4 cycles was optional [see Clinical Studies (14.3)]. A total of 1161 patients received intravenous pembrolizumab 200 mg (n=580) or placebo (n=581) every 3 weeks. Patients were ineligible if they had active autoimmune disease, were on chronic immunosuppressive agents, or had a history of interstitial lung disease or pneumonitis.
The median duration of exposure to intravenous pembrolizumab was 11.7 months (range: 1 day to 18.9 months). Sixty-eight percent of patients in the intravenous pembrolizumab arm were exposed to intravenous pembrolizumab for ≥6 months.
The adverse reactions observed in KEYNOTE-091 were generally similar to those occurring in other patients with NSCLC receiving intravenous pembrolizumab as a single agent, with the exception of hypothyroidism (22%), hyperthyroidism (11%), and pneumonitis (7%). Two fatal adverse reactions of myocarditis occurred.
Malignant Pleural Mesothelioma (MPM)
First-line treatment of unresectable advanced or metastatic MPM with pemetrexed and platinum chemotherapy
The safety of intravenous pembrolizumab in combination with pemetrexed and platinum chemotherapy (either carboplatin or cisplatin) was investigated in KEYNOTE-483, a multicenter, open-label, randomized (1:1), active-controlled trial in patients with previously untreated, unresectable advanced or metastatic MPM [see Clinical Studies (14.4)]. A total of 473 patients received intravenous pembrolizumab 200 mg, pemetrexed, and platinum every 3 weeks for up to 6 cycles followed by intravenous pembrolizumab (n=241), or pemetrexed and platinum chemotherapy every 3 weeks for up to 6 cycles (n=232). Patients with autoimmune disease that required systemic therapy within 3 years of treatment or a medical condition that required immunosuppression were ineligible.
The median duration of exposure to intravenous pembrolizumab 200 mg every 3 weeks was 6.9 months (range: 1 day to 25.2 months). Sixty-one percent of patients in the intravenous pembrolizumab arm were exposed to intravenous pembrolizumab for ≥6 months.
Adverse reactions occurring in patients with MPM were generally similar to those in other patients receiving intravenous pembrolizumab in combination with pemetrexed and platinum chemotherapy.
HNSCC
Neoadjuvant and Adjuvant Treatment of Locally Advanced HNSCC
The safety of intravenous pembrolizumab as neoadjuvant and adjuvant treatment added to standard of care (SOC) therapy was evaluated in KEYNOTE-689, a multicenter, randomized (1:1), open-label, active-controlled trial in patients with resectable locally advanced (Stage III-IVA by AJCC 8th edition) head and neck squamous cell carcinoma (HNSCC) [see Clinical Studies (14.5)]. Intravenous pembrolizumab was administered as single-agent neoadjuvant therapy before definitive surgery, during adjuvant radiotherapy (RT) with or without concurrent cisplatin, and then as single agent adjuvant therapy. Concurrent cisplatin was added to intravenous pembrolizumab and adjuvant RT for high-risk disease pathology. A total of 361 patients received treatment on the intravenous pembrolizumab arm and 315 patients received treatment on the SOC therapy arm.
The median duration of exposure to intravenous pembrolizumab in the neoadjuvant phase was 3.1 weeks (range: 1 day to 4.9 weeks). The median duration of exposure to intravenous pembrolizumab in the adjuvant phase was 42 weeks (range: 1 day to 82 weeks).
The median age of patients who received intravenous pembrolizumab was 60 years (range: 29 to 82), 32% age 65 or older, 6% age 75 or older; 79% male; 78% White, 14% Asian, 2.2% Black or African American, 2% Other races, 2.7% unknown race; and 15% Hispanic or Latino.
The most common adverse reactions (≥ 20%) on the intravenous pembrolizumab arm were stomatitis (48%), radiation skin injury (40%), weight loss (36%), fatigue (33%), dysphagia (29%), constipation (27%), hypothyroidism (26%), nausea (24%), rash (22%), dry mouth (22%), diarrhea (22%), and musculoskeletal pain (22%).
Table 18 and Table 19 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-689.
Table 18: Adverse Reactions in ≥20% of Patients with HNSCC Who Received Intravenous Pembrolizumab in KEYNOTE-689 Adverse Reaction Intravenous Pembrolizumab
Neoadjuvant then adjuvant beginning with RT with or without
cisplatin
n=361Standard of Care
Adjuvant RT with or without cisplatin
n=315All Grades*
(%)Grade 3 or 4
(%)All Grades*
(%)Grade 3 or 4
(%)- * Graded per NCI CTCAE v4.03
- † Includes pharyngeal inflammation, cheilitis, oral mucosal erythema, mucosal inflammation, glossitis, mouth ulceration, tongue ulceration
- ‡ Includes colitis, enteritis, hemorrhagic diarrhea
- § Includes blood thyroid stimulating hormone increased
- ¶ Includes asthenia
- # Includes dermatitis, skin exfoliation, dermatitis acneiform, eczema, rash macular, rash maculo-papular, erythema multiforme, dermatitis exfoliative, urticarial dermatitis, eczema asteatotic, exfoliative rash, rash pruritic, rash pustular
- Þ Includes neck pain, arthralgia, pain in extremity, back pain, myalgia, bone pain, arthritis, non-cardiac chest pain, musculoskeletal chest pain, musculoskeletal stiffness, spinal pain
Gastrointestinal Stomatitis† 48 14 60 13 Dysphagia 29 12 32 11 Constipation 27 0.3 22 0.3 Nausea 24 1.9 28 2.9 Dry mouth 22 1.4 26 1.6 Diarrhea‡ 22 4.2 11 0.6 Injury, poisoning and procedural complications Radiation skin injury 40 4.2 48 5.7 Investigations Weight loss 36 14 27 10 Endocrine disorders Hypothyroidism§ 26 0 6 0 General Fatigue¶ 33 2.2 27 1.6 Skin and subcutaneous tissue disorders Rash# 22 1.9 10 1.9 Musculoskeletal and connective tissue disorders Musculoskeletal pain Þ 22 1.9 16 0.6 Table 19: Select Laboratory Abnormalities (≥20%) that Worsened from Baseline in Patients with HNSCC Who Received Intravenous Pembrolizumab in KEYNOTE-689 Laboratory Test* Intravenous Pembrolizumab
Neoadjuvant then adjuvant beginning
with radiation with or without cisplatinRadiation with or without cisplatin All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: Intravenous Pembrolizumab + CRT/RT (range: 342 to 357 patients) and CRT/RT (range: 300 to 309 patients)
- † Graded per NCI CTCAE v4.03
Hematology Lymphopenia 76 50 86 55 Anemia 75 12 74 12 Neutropenia 32 13 35 19 Thrombocytopenia 22 1.7 29 1.9 Chemistry Hyperglycemia 57 5.7 47 2.7 Hyponatremia 47 16 36 11 Increased ALT 42 6.2 37 2.0 Hypoalbuminemia 40 1.4 38 1.0 Increased AST 38 4.8 23 2.0 Hypomagnesemia 34 1.2 22 1.3 Hypokalemia 29 6.8 20 7.4 Hyperkalemia 29 3.4 24 2.3 Increased alkaline phosphatase 27 2.5 19 0.0 Hypocalcemia 27 2.6 26 3.3 Hypophosphatemia 23 6.1 15 7.0 Increased creatinine 22 2.0 27 2.9 Hypercalcemia 21 2.0 14 1.3 Neoadjuvant Phase of KEYNOTE-689
Of the 361 patients who received at least one dose of single agent intravenous pembrolizumab as neoadjuvant treatment, 11% of patients experienced serious adverse reactions. Serious adverse reactions that occurred in more than one patient were pneumonia (1.4%), tumor hemorrhage (0.8%), dysphagia (0.6%), immune mediated hepatitis (0.6%), cellulitis (0.6%), and dyspnea (0.6%). Fatal adverse reactions occurred in 1.1% of patients who received neoadjuvant intravenous pembrolizumab including respiratory failure, clostridium infection, septic shock, and myocardial infarction (one patient each).
Permanent discontinuation of intravenous pembrolizumab due to an adverse reaction occurred in 2.8% of patients who received intravenous pembrolizumab as neoadjuvant treatment. The most frequent adverse reaction which resulted in permanent discontinuation of neoadjuvant intravenous pembrolizumab in more than one patient was arthralgia (0.6%).
Of the 361 patients who received intravenous pembrolizumab as neoadjuvant treatment, 11% (n=38) did not receive surgery. Surgical cancellation on the intravenous pembrolizumab arm was due to disease progression in 4% (n=15), patient decision in 3% (n=10), adverse reactions in 1.4% (n=5), physician’s decision in 1.1% (n=4), unresectable tumor in 0.6% (n=2), loss of follow-up in 0.3% (n=1), and use of non-study anti-cancer therapy in 0.3% (n=1).
Of the 351 patients randomized to SOC, 12% (n=43) did not receive surgery. Surgical cancellation on the SOC arm was due to patient decision in 7% (n=24), physician’s decision in 2.3% (n=8), disease progression in 1.7% (n=6), and adverse reactions in 1.4% (n=5).
Of the 323 intravenous pembrolizumab-treated patients who received surgery, 1.2% (n=4) experienced delay of surgery (defined as on-study surgery occurring ≥ 9 weeks after initiation of neoadjuvant intravenous pembrolizumab) due to adverse reactions. Of the 307 patients randomized to SOC who received surgery on study, 0.3% (n=1) experienced delay of surgery (defined as surgery occurring ≥ 6 weeks after randomization) due to adverse reactions.
Among the intravenous pembrolizumab-treated patients who received surgery, 2.8% (n=9) did not receive adjuvant treatment due to adverse reactions. Among the SOC-treated patients who received surgery, 3.6% (n=11) did not receive adjuvant RT or chemoradiation due to adverse reactions.
Adjuvant Phase of KEYNOTE-689
A total of 275 patients in the intravenous pembrolizumab arm and 275 patients in the SOC arm started the adjuvant phase of treatment. On the intravenous pembrolizumab arm, 100 patients received intravenous pembrolizumab and cisplatin with concurrent RT, 154 patients received intravenous pembrolizumab alone with concurrent RT, 7 patients received cisplatin alone with concurrent RT, and 13 patients received RT alone. One patient received intravenous pembrolizumab alone. On the SOC arm, 139 patients received cisplatin with concurrent RT while 136 patients received RT alone. For the intravenous pembrolizumab arm, a total of 222 patients received single-agent intravenous pembrolizumab following RT.
Of the 255 patients who received at least one dose of intravenous pembrolizumab in the adjuvant phase, 38% experienced serious adverse reactions. The most frequent serious adverse reactions reported in ≥1% of intravenous pembrolizumab-treated patients were pneumonia (2.7%), pyrexia (2.4%), stomatitis (2.4%), acute kidney injury (2.0%), pneumonitis (1.6%), COVID-19 (1.2%), death not otherwise specified (1.2%), diarrhea (1.2%), dysphagia (1.2%), gastrostomy tube site complication (1.2%), and immune-mediated hepatitis (1.2%).
Fatal adverse reactions occurred in 5% including death not otherwise specified (1.2%), acute renal failure (0.4%), hypercalcemia (0.4%), pulmonary hemorrhage (0.4%), dysphagia/malnutrition (0.4%), mesenteric thrombosis (0.4%), sepsis (0.4%), pneumonia (0.4%), COVID-19 (0.4%), respiratory failure (0.4%), cardiovascular disorder (0.4%) and gastrointestinal hemorrhage (0.4%).
Permanent discontinuation of adjuvant intravenous pembrolizumab due to an adverse reaction occurred in 17% of patients. The most frequent (≥1%) adverse reactions that led to permanent discontinuation of adjuvant intravenous pembrolizumab were pneumonitis, colitis, immune-mediated hepatitis and death not otherwise specified.
Of the 275 patients who received SOC in the adjuvant phase, 23% experienced serious adverse reactions.
The most frequent serious adverse reactions reported in >1% of SOC-treated patients were pneumonia (3.6%) and acute kidney injury (3.3%). Fatal adverse reactions occurred in 4.7% including pneumonia (0.7%), septic shock (0.4%), death not otherwise specified (0.4%), sudden death (0.4%), myocardial infarction (0.4%), pancreatic neoplasm (0.4%) and other infections (2.9%).
First-line treatment of metastatic or unresectable, recurrent HNSCC
The safety of intravenous pembrolizumab, as a single agent and in combination with platinum (cisplatin or carboplatin) and FU chemotherapy, was investigated in KEYNOTE-048, a multicenter, open-label, randomized (1:1:1), active-controlled trial in patients with previously untreated, recurrent or metastatic HNSCC [see Clinical Studies (14.5)]. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. A total of 576 patients received intravenous pembrolizumab 200 mg every 3 weeks either as a single agent (n=300) or in combination with platinum and FU (n=276) every 3 weeks for 6 cycles followed by intravenous pembrolizumab, compared to 287 patients who received cetuximab weekly in combination with platinum and FU every 3 weeks for 6 cycles followed by cetuximab.
The median duration of exposure to intravenous pembrolizumab was 3.5 months (range: 1 day to 24.2 months) in the intravenous pembrolizumab single agent arm and was 5.8 months (range: 3 days to 24.2 months) in the combination arm. Seventeen percent of patients in the intravenous pembrolizumab single agent arm and 18% of patients in the combination arm were exposed to intravenous pembrolizumab for ≥12 months. Fifty-seven percent of patients receiving intravenous pembrolizumab in combination with chemotherapy started treatment with carboplatin.
Intravenous pembrolizumab was discontinued for adverse reactions in 12% of patients in the intravenous pembrolizumab single agent arm. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab were sepsis (1.7%) and pneumonia (1.3%). Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 31% of patients; the most common adverse reactions leading to interruption of intravenous pembrolizumab (≥2%) were pneumonia (2.3%), pneumonitis (2.3%), and hyponatremia (2%).
Intravenous pembrolizumab was discontinued for adverse reactions in 16% of patients in the combination arm. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab were pneumonia (2.5%), pneumonitis (1.8%), and septic shock (1.4%). Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 45% of patients; the most common adverse reactions leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (14%), thrombocytopenia (10%), anemia (6%), pneumonia (4.7%), and febrile neutropenia (2.9%).
Tables 20 and 21 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-048.
Table 20: Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-048 Intravenous Pembrolizumab
200 mg every 3 weeksIntravenous Pembrolizumab
200 mg every 3 weeks
Platinum
FUCetuximab
Platinum
FUAdverse Reaction n=300 n=276 n=287 All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.0
- † Includes fatigue, asthenia
- ‡ Includes diarrhea, colitis, hemorrhagic diarrhea, microscopic colitis
- § Includes dermatitis, dermatitis acneiform, dermatitis allergic, dermatitis bullous, dermatitis contact, dermatitis exfoliative, drug eruption, erythema, erythema multiforme, rash, erythematous rash, generalized rash, macular rash, maculo-papular rash, pruritic rash, seborrheic dermatitis
- ¶ Includes cough, productive cough
- # Includes dyspnea, exertional dyspnea
- Þ Includes pneumonia, atypical pneumonia, bacterial pneumonia, staphylococcal pneumonia, aspiration pneumonia, lower respiratory tract infection, lung infection, lung infection pseudomonal
- ß Includes peripheral sensory neuropathy, peripheral neuropathy, hypoesthesia, dysesthesia
- à Includes back pain, musculoskeletal chest pain, musculoskeletal pain, myalgia
General Fatigue† 33 4 49 11 48 8 Pyrexia 13 0.7 16 0.7 12 0 Mucosal inflammation 4.3 1.3 31 10 28 5 Gastrointestinal Constipation 20 0.3 37 0 33 1.4 Nausea 17 0 51 6 51 6 Diarrhea‡ 16 0.7 29 3.3 35 3.1 Vomiting 11 0.3 32 3.6 28 2.8 Dysphagia 8 2.3 12 2.9 10 2.1 Stomatitis 3 0 26 8 28 3.5 Skin Rash§ 20 2.3 17 0.7 70 8 Pruritus 11 0 8 0 10 0.3 Respiratory, Thoracic and Mediastinal Cough¶ 18 0.3 22 0 15 0 Dyspnea# 14 2.0 10 1.8 8 1.0 Endocrine Hypothyroidism 18 0 15 0 6 0 Metabolism and Nutrition Decreased appetite 15 1.0 29 4.7 30 3.5 Weight loss 15 2 16 2.9 21 1.4 Infections PneumoniaÞ 12 7 19 11 13 6 Nervous System Headache 12 0.3 11 0.7 8 0.3 Dizziness 5 0.3 10 0.4 13 0.3 Peripheral sensory neuropathyß 1 0 14 1.1 7 1 Musculoskeletal Myalgiaà 12 1.0 13 0.4 11 0.3 Neck pain 6 0.7 10 1.1 7 0.7 Psychiatric Insomnia 7 0.7 10 0 8 0 Table 21: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-048 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeksIntravenous Pembrolizumab
200 mg every 3 weeks
Platinum
FUCetuximab
Platinum
FUAll Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab/chemotherapy (range: 240 to 267 patients), intravenous pembrolizumab (range: 245 to 292 patients), cetuximab/chemotherapy (range: 249 to 282 patients).
- † Graded per NCI CTCAE v4.0
Hematology Lymphopenia 54 25 70 35 75 46 Anemia 52 7 89 29 79 20 Thrombocytopenia 12 3.8 73 18 76 18 Neutropenia 8 1.4 68 37 73 43 Chemistry Hyperglycemia 47 3.8 54 6 65 4.7 Hyponatremia 46 18 55 20 59 20 Hypoalbuminemia 44 3.5 46 3.9 49 1.1 Increased AST 28 3.1 25 1.9 37 3.6 Increased ALT 25 2.1 22 1.5 38 1.8 Increased alkaline
phosphatase25 2.1 26 1.1 33 1.1 Hypercalcemia 22 4.5 16 4.2 13 2.5 Hypocalcemia 22 1.0 32 3.8 58 6 Hyperkalemia 21 2.8 28 4.2 29 4.6 Hypophosphatemia 20 5 34 12 49 20 Hypokalemia 19 5 33 12 47 15 Increased creatinine 17 1.0 36 2.3 27 2.1 Hypomagnesemia 15 0.4 40 1.7 76 9 Previously treated recurrent or metastatic HNSCC
Among the 192 patients with HNSCC enrolled in KEYNOTE-012 [see Clinical Studies (14.5)], the median duration of exposure to intravenous pembrolizumab was 3.3 months (range: 1 day to 27.9 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible for KEYNOTE-012.
The study population characteristics were: median age of 60 years (range: 20 to 84), 35% age 65 or older; 83% male; and 77% White, 15% Asian, and 5% Black. Sixty-one percent of patients had two or more lines of therapy in the recurrent or metastatic setting, and 95% had prior radiation therapy. Baseline ECOG PS was 0 (30%) or 1 (70%) and 86% had M1 disease.
Intravenous pembrolizumab was discontinued due to adverse reactions in 17% of patients. Serious adverse reactions occurred in 45% of patients receiving intravenous pembrolizumab. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonia, dyspnea, confusional state, vomiting, pleural effusion, and respiratory failure. The incidence of adverse reactions, including serious adverse reactions, was similar between dosage regimens (10 mg/kg every 2 weeks or 200 mg every 3 weeks); therefore, summary safety results are provided in a pooled analysis. The most common adverse reactions (occurring in ≥20% of patients) were fatigue, decreased appetite, and dyspnea. Adverse reactions occurring in patients with HNSCC were generally similar to those occurring in 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent, with the exception of increased incidences of facial edema (10% all Grades; 2.1% Grades 3-4) and new or worsening hypothyroidism [see Warnings and Precautions (5.1)].
Urothelial Cancer
Patients with urothelial cancer in combination with enfortumab vedotin
The safety of intravenous pembrolizumab in combination with enfortumab vedotin was investigated in KEYNOTE-A39 in patients with locally advanced or metastatic urothelial cancer [see Clinical Studies (14.6)]. A total of 440 patients received intravenous pembrolizumab 200 mg on Day 1 and enfortumab vedotin 1.25 mg/kg on Days 1 and 8 of each 21-day cycle compared to 433 patients who received gemcitabine on Days 1 and 8 and investigator’s choice of cisplatin or carboplatin on Day 1 of each 21-day cycle. Among patients who received intravenous pembrolizumab and enfortumab vedotin, the median duration of exposure to intravenous pembrolizumab was 8.5 months (range: 9 days to 28.5 months).
Fatal adverse reactions occurred in 3.9% of patients treated with intravenous pembrolizumab in combination with enfortumab vedotin including acute respiratory failure (0.7%), pneumonia (0.5%), and pneumonitis/ILD (0.2%).
Serious adverse reactions occurred in 50% of patients receiving intravenous pembrolizumab in combination with enfortumab vedotin. Serious adverse reactions in ≥2% of patients receiving intravenous pembrolizumab in combination with enfortumab vedotin were rash (6%), acute kidney injury (5%), pneumonitis/ILD (4.5%), urinary tract infection (3.6%), diarrhea (3.2%), pneumonia (2.3%), pyrexia (2%), and hyperglycemia (2%).
Permanent discontinuation of intravenous pembrolizumab occurred in 27% of patients. The most common adverse reactions (≥2%) resulting in permanent discontinuation of intravenous pembrolizumab were pneumonitis/ILD (4.8%) and rash (3.4%).
Dose interruptions of intravenous pembrolizumab occurred in 61% of patients. The most common adverse reactions (≥2%) resulting in interruption of intravenous pembrolizumab were rash (17%), peripheral neuropathy (7%), COVID-19 (5%), diarrhea (4.3%), pneumonitis/ILD (3.6%), neutropenia (3.4%), fatigue (3%), alanine aminotransferase increased (2.7%), hyperglycemia (2.5%), pneumonia (2%), and pruritus (2%).
Tables 22 and 23 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in combination with enfortumab vedotin in KEYNOTE-A39.
Table 22: Adverse Reactions ≥20% (All Grades) in Patients Treated with Intravenous Pembrolizumab in Combination with Enfortumab Vedotin in KEYNOTE-A39 Adverse Reaction Intravenous Pembrolizumab in combination with Enfortumab Vedotin
n=440Chemotherapy
n=433All Grades*
%Grades 3-4
%All Grades*
%Grades 3-4
%- * Graded per NCI CTCAE v4.03
- † Includes multiple terms
Skin and subcutaneous tissue disorders Rash† 68 15 15 0 Pruritus 41 1.1 7 0 Alopecia 35 0.5 8 0.2 General disorders and administration site conditions Fatigue† 51 6 57 7 Nervous system disorders Peripheral neuropathy† 67 8 14 0 Dysgeusia 21 0 9 0 Metabolism and nutrition disorders Decreased appetite 33 1.8 26 1.8 Gastrointestinal disorders Diarrhea 38 4.5 16 1.4 Nausea 26 1.6 41 2.8 Constipation 26 0 34 0.7 Investigations Weight loss 33 3.6 9 0.2 Eye disorders Dry eye† 24 0 2.1 0 Infections and infestations Urinary tract infection 21 5 19 8 Clinically relevant adverse reactions (<20%) include pyrexia (18%), dry skin (17%), vomiting (12%), pneumonitis/ILD (10%), hypothyroidism (10%), blurred vision (6%), infusion site extravasation (2%), and myositis (0.5%).
Table 23: Selected Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients in KEYNOTE-A39 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks and
Enfortumab VedotinChemotherapy All Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 407 to 439 patients)
- † Graded per NCI CTCAE v4.03
Chemistry Increased aspartate aminotransferase 75 4.6 39 3.3 Increased creatinine 71 3.2 68 2.6 Hyperglycemia 66 14 54 4.7 Increased alanine aminotransferase 59 5 49 3.3 Hyponatremia 46 13 47 13 Hypophosphatemia 44 9 36 9 Hypoalbuminemia 39 1.8 35 0.5 Hypokalemia 26 5 16 3.1 Hyperkalemia 24 1.4 36 4.0 Hypercalcemia 21 1.2 14 0.2 Hematology Lymphopenia 58 15 59 17 Anemia 53 7 89 33 Neutropenia 30 9 80 50 Cisplatin-ineligible patients with urothelial cancer in combination with enfortumab vedotin
The safety of intravenous pembrolizumab in combination with enfortumab vedotin was investigated in KEYNOTE-869 in patients with locally advanced or metastatic urothelial cancer and who are not eligible for cisplatin-based chemotherapy [see Clinical Studies (14.6)]. A total of 121 patients received intravenous pembrolizumab 200 mg on Day 1, and enfortumab vedotin 1.25 mg/kg on days 1 and 8 of each 21-day cycle. The median duration of exposure to intravenous pembrolizumab was 6.9 months (range 1 day to 29.6 months).
Fatal adverse reactions occurred in 5% of patients treated with intravenous pembrolizumab in combination with enfortumab vedotin, including sepsis (1.6%), bullous dermatitis (0.8%), myasthenia gravis (0.8%), and pneumonitis (0.8%).
Serious adverse reactions occurred in 50% of patients receiving intravenous pembrolizumab and enfortumab vedotin. Serious adverse reactions in ≥2% of patients receiving intravenous pembrolizumab in combination with enfortumab vedotin were acute kidney injury (7%), urinary tract infection (7%), urosepsis (5%), hematuria (3.3%), pneumonia (3.3%), pneumonitis (3.3%), sepsis (3.3%), anemia (2.5%), diarrhea (2.5%), hypotension (2.5%), myasthenia gravis (2.5%), myositis (2.5%), and urinary retention (2.5%).
Permanent discontinuation of intravenous pembrolizumab occurred in 32% of patients. The most common adverse reactions (≥2%) resulting in permanent discontinuation of intravenous pembrolizumab were pneumonitis (5%), peripheral neuropathy (5%), rash (3.3%), and myasthenia gravis (2.5%).
Dose interruptions of intravenous pembrolizumab occurred in 69% of patients. The most common adverse reactions (≥2%) resulting in interruption of intravenous pembrolizumab were peripheral neuropathy (22%), rash (17%), neutropenia (7%), fatigue (6%), diarrhea (5%), lipase increased (5%), acute kidney injury (3.3%), ALT increased (2.5%), and COVID-19 (2.5%).
Tables 24 and 25 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in combination with enfortumab vedotin in KEYNOTE-869.
Table 24: Adverse Reactions Occurring in ≥20% of Patients Treated with Intravenous Pembrolizumab in Combination with Enfortumab Vedotin in KEYNOTE-869 Adverse Reaction Intravenous Pembrolizumab in combination with Enfortumab Vedotin
n=121All Grades*
%Grade 3-4
%- * Graded per NCI CTCAE v4.03
- † Includes: blister, conjunctivitis, dermatitis, dermatitis bullous, dermatitis exfoliative generalized, erythema, erythema multiforme, exfoliative rash, palmar-plantar erythrodysesthesia syndrome, pemphigoid, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic, rash vesicular, skin exfoliation, and stomatitis
- ‡ Includes: dysesthesia, hypoesthesia, muscular weakness, paresthesia, peripheral motor neuropathy, peripheral sensorimotor neuropathy, peripheral sensory neuropathy, and gait disturbance
Skin and subcutaneous tissue disorders Rash† 71 21 Alopecia 52 0 Pruritus 40 3.3 Dry skin 21 0.8 Nervous system disorders Peripheral neuropathy‡ 65 3.3 Dysgeusia 35 0 Dizziness 23 0 General disorders and administration site conditions Fatigue 60 11 Peripheral edema 26 0 Investigations Weight loss 48 5 Gastrointestinal disorders Diarrhea 45 7 Nausea 36 0.8 Constipation 27 0 Metabolism and nutrition disorders Decreased appetite 38 0.8 Infections and infestations Urinary tract infection 30 12 Eye disorders Dry eye 25 0 Musculoskeletal and connective tissue disorders Arthralgia 23 1.7 Clinically relevant adverse reactions (<20%) include vomiting (19.8%), fever (18%), hypothyroidism (11%), pneumonitis/ILD (10%), myositis (3.3%), myasthenia gravis (2.5%), and infusion site extravasation (0.8%).
Table 25: Selected Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients in KEYNOTE-869 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks and
Enfortumab VedotinAll Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 114 to 121 patients)
- † Graded per NCI CTCAE v4.03
Chemistry Hyperglycemia 74 13 Increased aspartate aminotransferase 73 9 Increased creatinine 69 3.3 Hyponatremia 60 19 Increased alanine aminotransferase 60 7 Increased lipase 59 32 Hypoalbuminemia 59 4.2 Hypophosphatemia 51 15 Hypokalemia 35 8 Increased potassium 27 1.7 Increased calcium 27 4.2 Hematology Anemia 69 15 Lymphopenia 64 17 Neutropenia 32 12 Platinum-Ineligible Patients with Urothelial Carcinoma
The safety of intravenous pembrolizumab was investigated in KEYNOTE-052, a single-arm trial that enrolled 370 patients with locally advanced or metastatic urothelial carcinoma who had one or more comorbidities. Patients with autoimmune disease or medical conditions that required systemic corticosteroids or other immunosuppressive medications were ineligible [see Clinical Studies (14.6)]. Patients received intravenous pembrolizumab 200 mg every 3 weeks until unacceptable toxicity or either radiographic or clinical disease progression.
The median duration of exposure to intravenous pembrolizumab was 2.8 months (range: 1 day to 15.8 months).
Intravenous pembrolizumab was discontinued due to adverse reactions in 11% of patients. Eighteen patients (5%) died from causes other than disease progression. Five patients (1.4%) who were treated with intravenous pembrolizumab experienced sepsis which led to death, and three patients (0.8%) experienced pneumonia which led to death. Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 22% of patients; the most common (≥1%) were liver enzyme increase, diarrhea, urinary tract infection, acute kidney injury, fatigue, joint pain, and pneumonia. Serious adverse reactions occurred in 42% of patients. The most frequent serious adverse reactions (≥2%) were urinary tract infection, hematuria, acute kidney injury, pneumonia, and urosepsis.
Immune-related adverse reactions that required systemic glucocorticoids occurred in 8% of patients, use of hormonal supplementation due to an immune-related adverse reaction occurred in 8% of patients, and 5% of patients required at least one steroid dose ≥40 mg oral prednisone equivalent.
Table 26 summarizes adverse reactions in patients on intravenous pembrolizumab in KEYNOTE-052.
Table 26: Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-052 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
N=370All Grades*
(%)Grades 3–4
(%)- * Graded per NCI CTCAE v4.0
- † Includes fatigue, asthenia
- ‡ Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, pain in extremity, spinal pain
- § Includes diarrhea, colitis, enterocolitis, gastroenteritis, frequent bowel movements
- ¶ Includes abdominal pain, pelvic pain, flank pain, abdominal pain lower, tumor pain, bladder pain, hepatic pain, suprapubic pain, abdominal discomfort, abdominal pain upper
- # Includes autoimmune hepatitis, hepatitis, hepatitis toxic, liver injury, increased transaminases, hyperbilirubinemia, increased blood bilirubin, increased alanine aminotransferase, increased aspartate aminotransferase, increased hepatic enzymes, increased liver function tests
- Þ Includes dermatitis, dermatitis bullous, eczema, erythema, rash, rash macular, rash maculo-papular, rash pruritic, rash pustular, skin reaction, dermatitis acneiform, seborrheic dermatitis, palmar-plantar erythrodysesthesia syndrome, rash generalized
- ß Includes edema peripheral, peripheral swelling
General Fatigue† 38 6 Pyrexia 11 0.5 Weight loss 10 0 Musculoskeletal and Connective Tissue Musculoskeletal pain‡ 24 4.9 Arthralgia 10 1.1 Metabolism and Nutrition Decreased appetite 22 1.6 Hyponatremia 10 4.1 Gastrointestinal Constipation 21 1.1 Diarrhea§ 20 2.4 Nausea 18 1.1 Abdominal pain¶ 18 2.7 Elevated LFTs# 13 3.5 Vomiting 12 0 Skin and Subcutaneous Tissue RashÞ 21 0.5 Pruritus 19 0.3 Edema peripheralß 14 1.1 Infections Urinary tract infection 19 9 Blood and Lymphatic System Anemia 17 7 Respiratory, Thoracic, and Mediastinal Cough 14 0 Dyspnea 11 0.5 Renal and Urinary Increased blood creatinine 11 1.1 Hematuria 13 3.0 Previously Treated Urothelial Carcinoma
The safety of intravenous pembrolizumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma with disease progression following platinum-containing chemotherapy was investigated in KEYNOTE-045. KEYNOTE-045 was a multicenter, open-label, randomized (1:1), active-controlled trial in which 266 patients received intravenous pembrolizumab 200 mg every 3 weeks or investigator's choice of chemotherapy (n=255), consisting of paclitaxel (n=84), docetaxel (n=84) or vinflunine (n=87) [see Clinical Studies (14.6)]. Patients with autoimmune disease or a medical condition that required systemic corticosteroids or other immunosuppressive medications were ineligible.
The median duration of exposure was 3.5 months (range: 1 day to 20 months) in patients who received intravenous pembrolizumab and 1.5 months (range: 1 day to 14 months) in patients who received chemotherapy.
Intravenous pembrolizumab was discontinued due to adverse reactions in 8% of patients. The most common adverse reaction resulting in permanent discontinuation of intravenous pembrolizumab was pneumonitis (1.9%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 20% of patients; the most common (≥1%) were urinary tract infection (1.5%), diarrhea (1.5%), and colitis (1.1%). Serious adverse reactions occurred in 39% of intravenous pembrolizumab-treated patients. The most frequent serious adverse reactions (≥2%) in intravenous pembrolizumab-treated patients were urinary tract infection, pneumonia, anemia, and pneumonitis. Tables 27 and 28 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-045.
Table 27: Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-045 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
n=266Chemotherapy*
n=255All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Chemotherapy: paclitaxel, docetaxel, or vinflunine
- † Graded per NCI CTCAE v4.0
- ‡ Includes asthenia, fatigue, malaise, lethargy
- § Includes back pain, myalgia, bone pain, musculoskeletal pain, pain in extremity, musculoskeletal chest pain, musculoskeletal discomfort, neck pain
- ¶ Includes rash maculo-papular, rash, genital rash, rash erythematous, rash papular, rash pruritic, rash pustular, erythema, drug eruption, eczema, eczema asteatotic, dermatitis contact, dermatitis acneiform, dermatitis, seborrheic keratosis, lichenoid keratosis
- # Includes diarrhea, gastroenteritis, colitis, enterocolitis
- Þ Includes cough, productive cough
- ß Includes dyspnea, dyspnea exertional, wheezing
- à Includes blood urine present, hematuria, chromaturia
General Fatigue‡ 38 4.5 56 11 Pyrexia 14 0.8 13 1.2 Musculoskeletal and Connective Tissue Musculoskeletal pain§ 32 3.0 27 2.0 Skin and Subcutaneous Tissue Pruritus 23 0 6 0.4 Rash¶ 20 0.4 13 0.4 Gastrointestinal Nausea 21 1.1 29 1.6 Constipation 19 1.1 32 3.1 Diarrhea# 18 2.3 19 1.6 Vomiting 15 0.4 13 0.4 Abdominal pain 13 1.1 13 2.7 Metabolism and Nutrition Decreased appetite 21 3.8 21 1.2 Infections Urinary tract infection 15 4.9 14 4.3 Respiratory, Thoracic and Mediastinal CoughÞ 15 0.4 9 0 Dyspneaß 14 1.9 12 1.2 Renal and Urinary Hematuria à 12 2.3 8 1.6 Table 28: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Urothelial Carcinoma Patients Receiving Intravenous Pembrolizumab in KEYNOTE-045 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeksChemotherapy All Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 240 to 248 patients) and chemotherapy (range: 238 to 244 patients); phosphate decreased: intravenous pembrolizumab n=232 and chemotherapy n=222.
- † Graded per NCI CTCAE v4.0
Chemistry Hyperglycemia 52 8 60 7 Anemia 52 13 68 18 Lymphopenia 45 15 55 26 Hypoalbuminemia 43 1.7 50 3.8 Hyponatremia 37 9 47 13 Increased alkaline phosphatase 37 7 33 4.9 Increased creatinine 35 4.4 28 2.9 Hypophosphatemia 29 8 34 14 Increased AST 28 4.1 20 2.5 Hyperkalemia 28 0.8 27 6 Hypocalcemia 26 1.6 34 2.1 Neoadjuvant and Adjuvant Treatment of Cisplatin-Ineligible Patients with MIBC in Combination with Enfortumab Vedotin
The safety of intravenous pembrolizumab in combination with enfortumab vedotin as neoadjuvant treatment and continued after radical cystectomy (RC) as adjuvant treatment was investigated in KEYNOTE-905, an open-label, multicenter, randomized, active-controlled trial in patients with previously untreated MIBC who were ineligible for or declined cisplatin-based chemotherapy. Patients received intravenous pembrolizumab in combination with enfortumab vedotin (n=167) before and after RC with pelvic lymph node dissection (PLND) or RC with PLND alone (n=159) [see Clinical Studies (14.7)].
For the 167 patients who received intravenous pembrolizumab in the neoadjuvant phase, the median duration of exposure to intravenous pembrolizumab 200 mg every 3 weeks was 1.4 months (range: 1 day to 2.7 months) and the median number of cycles of intravenous pembrolizumab was 3 (range: 1 to 3) out of the planned 3 cycles in the neoadjuvant phase. For the 96 patients who received intravenous pembrolizumab in the adjuvant phase, the median duration of exposure to intravenous pembrolizumab 200 mg every 3 weeks was 8.5 months (range: 1 day to 12.9 months) and the median number of cycles of intravenous pembrolizumab was 12 (range: 1 to 14) out of the planned 14 cycles in the adjuvant phase. Across the combined neoadjuvant and adjuvant phases (n=167), the median number of cycles of intravenous pembrolizumab was 5 (range: 1 to 17) out of the planned 17 cycles.
Tables 29 and 30 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in combination with enfortumab vedotin in KEYNOTE-905.
Table 29: Adverse Reactions ≥20% (All Grades) in Patients Treated with Intravenous Pembrolizumab in Combination with Enfortumab Vedotin in KEYNOTE-905 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks in combination with
enfortumab vedotin before and after RC with
PLND
n=167
RC with PLND alone
n=159All Grades
%Grade 3-4
%All Grades
%Grade 3-4
%- * Includes multiple terms.
- † Includes rash, rash maculo-papular, conjunctivitis, erythema, eczema, skin exfoliation, palmar-plantar erythrodysesthesia syndrome, blister, dermatitis, dermatitis exfoliative generalized, exfoliative rash, rash papular, rash pruritic, dermatitis bullous, drug eruption, pemphigoid, rash vesicular, and dermatitis contact.
Skin and subcutaneous tissue disorders Rash*, † 54 7 1.3 0 Pruritus 47 3 0 0 Alopecia 35 0.6 0 0 General disorders and administration site conditions Fatigue* 47 4.2 6 0.6 Nervous system disorders Peripheral neuropathy* 39 3 1.9 0 Dysgeusia* 35 0 0 0 Gastrointestinal disorders Diarrhea* 34 5 3.1 1.3 Constipation 28 1.8 8 0 Nausea 26 1.2 8 0.6 Metabolism and nutrition disorders Decreased appetite 28 0.6 1.9 0 Infections and infestations Urinary tract infection 24 12 13 11 Eye disorders Dry eye* 21 0 0 0 Investigations Weight loss 20 0 3.1 0 Clinically relevant adverse reactions (<20%) include dry skin (15%), hypothyroidism (14%), vomiting (9%), pneumonitis/ILD (4.2%), skin hyperpigmentation (3.0%), infusion site extravasation (1.2%), and myasthenia gravis and myositis (0.6% each).
Table 30: Selected Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Treated with Intravenous Pembrolizumab in Combination with Enfortumab Vedotin in KEYNOTE-905 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks in
combination with enfortumab
vedotin before and after RC with
PLND
n=167RC with PLND alone
n=159All Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%ALT = alanine aminotransferase; AST = aspartate aminotransferase. - * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: Intravenous pembrolizumab in combination with enfortumab vedotin (167 patients), and RC and PLND alone (range: 110 to 121 patients).
- † Graded per NCI CTCAE v4.03
Chemistry Increased glucose 72 12 24 1.7 Increased AST 55 6 11 1.8 Increased ALT 53 4.8 13 0.9 Increased creatinine 47 8 31 2.5 Decreased sodium 44 13 18 7 Increased potassium 39 7 20 6 Decreased phosphate 26 6 1.8 0 Hematology Decreased hemoglobin 60 13 48 8 Decreased lymphocytes 40 8 17 1.7 Neoadjuvant Phase of KEYNOTE-905
A total of 167 patients received at least 1 dose of intravenous pembrolizumab in combination with enfortumab vedotin as neoadjuvant treatment before receiving RC.
In the neoadjuvant phase, serious adverse reactions occurred in 27% of patients receiving intravenous pembrolizumab in combination with enfortumab vedotin. The most frequent (≥2%) serious adverse reactions were urinary tract infection (3.6%) and hematuria (2.4%). Fatal adverse reactions occurred in 1.2% of patients, including myasthenia gravis and toxic epidermal necrolysis (0.6% each). Additional fatal adverse reactions were reported in 2.7% of patients in the post-surgery phase before adjuvant treatment started, including sepsis and intestinal obstruction (1.4% each).
Permanent discontinuation of intravenous pembrolizumab due to an adverse reaction occurred in 15% of patients. The most frequent (>1%) adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab were rash (2.4%, including generalized exfoliative dermatitis), increased alanine aminotransferase, increased aspartate aminotransferase, diarrhea, dysgeusia, and toxic epidermal necrolysis (1.2% each).
Adverse reactions leading to dose interruption of intravenous pembrolizumab in the neoadjuvant phase occurred in 20% of patients. The most common adverse reactions (≥2%) leading to dose interruption of intravenous pembrolizumab were rash (4.8%) and neutropenia (2.4%). Of the 167 patients in the intravenous pembrolizumab in combination with enfortumab vedotin arm who received neoadjuvant treatment, 7 (4.2%) patients did not receive surgery due to adverse reactions. The adverse reactions that led to cancellation of surgery were acute myocardial infarction, bile duct cancer, colon cancer, respiratory distress, urinary tract infection, and the two deaths due to myasthenia gravis and toxic epidermal necrolysis (0.6% each).
Of the 146 patients who received neoadjuvant treatment with intravenous pembrolizumab in combination with enfortumab vedotin and underwent radical cystectomy, 6 (4.1%) patients experienced delay of surgery (defined as time from last neoadjuvant treatment to surgery exceeding 8 weeks) due to adverse reactions.
Adjuvant Phase of KEYNOTE-905
Patients who did not proceed to surgery were ineligible for adjuvant therapy. Of the 149 patients who underwent surgery, 100 patients received adjuvant treatment with intravenous pembrolizumab in combination with enfortumab vedotin. Of the 49 patients who did not receive adjuvant treatment, discontinuation of treatment prior to the adjuvant phase was due to an adverse event in 21 patients.
In the adjuvant phase, serious adverse reactions occurred in 43% of patients; the most frequent (≥2%) serious adverse reactions were urinary tract infection (8%), acute kidney injury and pyelonephritis (5% each), urosepsis (4%), and hypokalemia, intestinal obstruction, and sepsis (2% each). Fatal adverse reactions occurred in 7% of patients, including urosepsis, intracranial hemorrhage, death, myocardial infarction, multiple organ dysfunction syndrome, and pseudomonal pneumonia (1% each).
Permanent discontinuation of intravenous pembrolizumab due to an adverse reaction occurred in 28% of patients. The most frequent (>1%) adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab were diarrhea (5%), and peripheral neuropathy, acute kidney injury, and pneumonitis (2% each).
Adverse reactions leading to dose interruption of intravenous pembrolizumab in the adjuvant phase occurred in 38% of patients. The most common adverse reactions (≥2%) leading to dose interruption of intravenous pembrolizumab were rash (7%), urinary tract infection (6%), diarrhea (4%), and abdominal pain, COVID-19, fatigue, pruritus, and pyelonephritis (2% each).
BCG-unresponsive High-risk NMIBC
The safety of intravenous pembrolizumab was investigated in KEYNOTE-057, a multicenter, open-label, single-arm trial that enrolled 148 patients with high-risk non-muscle invasive bladder cancer (NMIBC), 96 of whom had BCG-unresponsive carcinoma in situ (CIS) with or without papillary tumors. Patients received intravenous pembrolizumab 200 mg every 3 weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC or progressive disease, or up to 24 months of therapy without disease progression.
The median duration of exposure to intravenous pembrolizumab was 4.3 months (range: 1 day to 25.6 months).
Intravenous pembrolizumab was discontinued due to adverse reactions in 11% of patients. The most common adverse (>1%) reaction resulting in permanent discontinuation of intravenous pembrolizumab was pneumonitis (1.4%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 22% of patients; the most common (≥2%) were diarrhea (4%) and urinary tract infection (2%). Serious adverse reactions occurred in 28% of intravenous pembrolizumab-treated patients. The most frequent serious adverse reactions (≥2%) in intravenous pembrolizumab-treated patients were pneumonia (3%), cardiac ischemia (2%), colitis (2%), pulmonary embolism (2%), sepsis (2%), and urinary tract infection (2%). Tables 31 and 32 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-057.
Table 31: Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-057 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
N=148All Grades*
(%)Grades 3–4
(%)- * Graded per NCI CTCAE v4.03
- † Includes asthenia, fatigue, malaise
- ‡ Includes edema peripheral, peripheral swelling
- § Includes diarrhea, gastroenteritis, colitis
- ¶ Includes rash maculo-papular, rash, rash erythematous, rash pruritic, rash pustular, erythema, eczema, eczema asteatotic, lichenoid keratosis, urticaria, dermatitis
- # Includes back pain, myalgia, musculoskeletal pain, pain in extremity, musculoskeletal chest pain, neck pain
- Þ Includes cough, productive cough
General Fatigue† 29 0.7 Peripheral edema‡ 11 0 Gastrointestinal Diarrhea§ 24 2.0 Nausea 13 0 Constipation 12 0 Skin and Subcutaneous Tissue Rash¶ 24 0.7 Pruritus 19 0.7 Musculoskeletal and Connective Tissue Musculoskeletal pain# 19 0 Arthralgia 14 1.4 Renal and Urinary Hematuria 19 1.4 Respiratory, Thoracic, and Mediastinal CoughÞ 19 0 Infections Urinary tract infection 12 2.0 Nasopharyngitis 10 0 Endocrine Hypothyroidism 11 0 Table 32: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of BCG-unresponsive NMIBC Patients Receiving Intravenous Pembrolizumab in KEYNOTE-057 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeksAll Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 124 to 147 patients)
- † Graded per NCI CTCAE v4.03
Chemistry Hyperglycemia 59 7 Increased ALT 25 2.7 Hyponatremia 24 7 Hypophosphatemia 24 6 Hypoalbuminemia 24 1.4 Hyperkalemia 23 1.4 Hypocalcemia 22 0.7 Increased AST 20 2.7 Increased creatinine 20 0.7 Hematology Anemia 35 1.4 Lymphopenia 29 1.6 Microsatellite Instability-High or Mismatch Repair Deficient Cancer
The safety of intravenous pembrolizumab was investigated in 504 patients with MSI-H or dMMR cancer enrolled in KEYNOTE-158, KEYNOTE-164, and KEYNOTE-051 [see Clinical Studies (14.7)]. The median duration of exposure to intravenous pembrolizumab was 6.2 months (range: 1 day to 53.5 months). Adverse reactions occurring in patients with MSI-H or dMMR cancer were similar to those occurring in patients with other solid tumors who received intravenous pembrolizumab as a single agent.
Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer
Among the 153 patients with MSI-H or dMMR CRC enrolled in KEYNOTE-177 [see Clinical Studies (14.8)] treated with intravenous pembrolizumab, the median duration of exposure to intravenous pembrolizumab was 11.1 months (range: 1 day to 30.6 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Adverse reactions occurring in patients with MSI-H or dMMR CRC were similar to those occurring in 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent.
Gastric Cancer
First-line Treatment of Locally Advanced Unresectable or Metastatic HER2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma
The safety of intravenous pembrolizumab was evaluated in 696 patients with HER2-positive gastric or GEJ cancer enrolled in KEYNOTE-811, which included 350 patients treated with intravenous pembrolizumab 200 mg, trastuzumab, and CAPOX (n=297) or FP (n=53) every 3 weeks, compared to 346 patients treated with placebo, trastuzumab, and CAPOX (n=298) or FP (n=48) every 3 weeks [see Clinical Studies (14.10)].
The median duration of exposure to intravenous pembrolizumab was 9.2 months (range: 1 day to 33.6 months).
Fatal adverse reactions occurred in 3 patients who received intravenous pembrolizumab in combination with trastuzumab and CAPOX or FP and included pneumonitis in 2 patients and hepatitis in 1 patient.
Intravenous pembrolizumab was discontinued due to adverse reactions in 13% of patients. Adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab in ≥1% of patients were pneumonitis (2.0%) and pneumonia (1.1%).
Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 71% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (21%), thrombocytopenia (13%), diarrhea (7%), pneumonia (5%), anemia (4.9%), COVID-19 (3.1%), hypokalemia (3.1%), fatigue/asthenia (4.9%), decreased appetite (4%), increased AST (3.7%), increased blood bilirubin (4.6%), increased ALT (2.9%), vomiting (2.6%), pneumonitis (2.3%), pyrexia (2.3%), increased blood creatinine (2%), and colitis (2%).
In the intravenous pembrolizumab arm versus placebo, there was a difference of ≥5% incidence between patients treated with intravenous pembrolizumab versus standard of care for diarrhea (53% vs. 47%), rash (35% vs. 28%), hypothyroidism (11% vs. 5%), and pneumonia (11% vs. 5%). There were no clinically meaningful differences in incidence of Grade 3-4 toxicity between arms.
There was a difference of ≥5% incidence between patients treated with intravenous pembrolizumab versus standard of care for decreased leukocytes (60% vs. 54%), decreased calcium (56% vs. 46%), decreased lymphocytes (59% vs. 51%), decreased potassium (41% vs. 36%), increased bilirubin (33% vs. 25%), increased creatinine (28% vs. 18%), and decreased glucose (17% vs. 11%). There were no clinically meaningful differences in incidence of Grade 3-4 toxicity between arms.
First-line Treatment of Locally Advanced Unresectable or Metastatic HER2-Negative Gastric or Gastroesophageal Junction Adenocarcinoma
The safety of intravenous pembrolizumab was evaluated in 1572 patients with HER2-negative gastric or GEJ cancer enrolled in KEYNOTE-859, which included 785 patients treated with intravenous pembrolizumab 200 mg and FP (n=106) or CAPOX (n=674) every 3 weeks, compared to 787 patients who received placebo and FP (n=107) or CAPOX (n=679) every 3 weeks [see Clinical Studies (14.9)].
The median duration of exposure to intravenous pembrolizumab was 6.2 months (range: 1 day to 33.7 months).
Serious adverse reactions occurred in 45% of patients receiving intravenous pembrolizumab. Serious adverse reactions in >2% of patients included pneumonia (4.1%), diarrhea (3.9%), hemorrhage (3.9%), and vomiting (2.4%). Fatal adverse reactions occurred in 8% of patients who received intravenous pembrolizumab, including infection (2.3%) and thromboembolism (1.3%).
Permanent discontinuation of intravenous pembrolizumab due to adverse reactions occurred in 15% of patients. Adverse reaction resulting in permanent discontinuation of intravenous pembrolizumab in ≥1% were infections (1.8%) and diarrhea (1.0%).
Dosage interruptions of intravenous pembrolizumab due to an adverse reaction occurred in 65% of patients. Adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (21%), thrombocytopenia (13%), diarrhea (5.5%), fatigue (4.8%), infection (4.8%), anemia (4.5%), increased AST (4.3%), increased ALT (3.8%), increased blood bilirubin (3.3%), white blood cell count decreased (2.2%), nausea (2%), palmar-plantar erythrodysesthesia syndrome (2%), and vomiting (2%).
Tables 33 and 34 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-859.
Table 33: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-859 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
and FP or CAPOX
n=785Placebo
and FP or CAPOX
n=787All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes dysesthesia, hyperesthesia, hypoesthesia, neuralgia, neuropathy peripheral, paresthesia, peripheral sensory neuropathy, peripheral motor neuropathy, polyneuropathy
- ‡ Includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal tenderness, abdominal pain upper, epigastric discomfort, gastrointestinal pain
- § Includes asthenia, fatigue
Nervous System Peripheral neuropathy† 47 5 48 6 Gastrointestinal Nausea 46 3.7 46 4.4 Diarrhea 36 6 32 5 Vomiting 34 5 27 5 Abdominal Pain‡ 26 2.8 24 2.9 Constipation 22 0.5 21 0.8 General Fatigue§ 40 8 39 9 Metabolism and Nutrition Decreased appetite 29 3.3 29 2.5 Skin and Subcutaneous Tissue Palmar-plantar erythrodysesthesia syndrome 25 3.1 22 1.8 Investigations Weight loss 20 2.8 19 2.7 Table 34: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-859 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
and FP or CAPOXPlacebo
and FP or CAPOXAll Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab/FP or CAPOX (range: 210 to 766 patients) and placebo/FP or CAPOX (range: 190 to 762 patients)
- † Graded per NCI CTCAE v4.03
Hematology Anemia 65 15 69 13 Thrombocytopenia 64 12 62 10 Neutropenia 63 25 58 20 Leukopenia 59 7 56 6 Lymphopenia 57 20 51 16 Chemistry Increased AST 57 4.7 48 3.6 Hypoalbuminemia 55 4.1 52 2.9 Hyperglycemia 53 6 52 4.6 Hypocalcemia 49 3.6 45 3.3 Increased alkaline phosphatase 48 6 41 5 Hyponatremia 40 13 40 12 Increased ALT 40 4.2 29 2.9 Hypokalemia 35 10 27 9 Bilirubin increased 32 5 30 5 Hypophosphatemia 30 10 27 8 Hypomagnesemia 29 0.3 22 0.7 Increased creatinine 21 3.5 18 1.7 Hyperkalemia 20 3.7 18 2.9 Increased INR 20 1.4 22 0 Esophageal Cancer
First-line Treatment of Locally Advanced Unresectable or Metastatic Esophageal Cancer/Gastroesophageal Junction
The safety of intravenous pembrolizumab, in combination with cisplatin and FU chemotherapy was investigated in KEYNOTE-590, a multicenter, double-blind, randomized (1:1), placebo-controlled trial for the first-line treatment in patients with metastatic or locally advanced esophageal or gastroesophageal junction (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma who were not candidates for surgical resection or definitive chemoradiation [see Clinical Studies (14.10)]. A total of 740 patients received either intravenous pembrolizumab 200 mg (n=370) or placebo (n=370) every 3 weeks for up to 35 cycles, both in combination with up to 6 cycles of cisplatin and up to 35 cycles of FU.
The median duration of exposure was 5.7 months (range: 1 day to 26 months) in the intravenous pembrolizumab combination arm and 5.1 months (range: 3 days to 27 months) in the chemotherapy arm.
Intravenous pembrolizumab was discontinued for adverse reactions in 15% of patients. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab (≥1%) were pneumonitis (1.6%), acute kidney injury (1.1%), and pneumonia (1.1%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 67% of patients. The most common adverse reactions leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (19%), fatigue/asthenia (8%), decreased white blood cell count (5%), pneumonia (5%), decreased appetite (4.3%), anemia (3.2%), increased blood creatinine (3.2%), stomatitis (3.2%), malaise (3.0%), thrombocytopenia (3%), pneumonitis (2.7%), diarrhea (2.4%), dysphagia (2.2%), and nausea (2.2%).
Tables 35 and 36 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-590.
Table 35: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-590 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
Cisplatin
FU
n=370Placebo
Cisplatin
FU
n=370All Grades*
(%)Grades 3-4†
(%)All Grades*
(%)Grades 3-4†
(%)- * Graded per NCI CTCAE v4.03
- † One fatal event of diarrhea was reported in each arm.
- ‡ Includes asthenia, fatigue
Gastrointestinal Nausea 67 7 63 7 Constipation 40 0 40 0 Diarrhea 36 4.1 33 3 Vomiting 34 7 32 5 Stomatitis 27 6 26 3.8 General Fatigue‡ 57 12 46 9 Metabolism and Nutrition Decreased appetite 44 4.1 38 5 Investigations Weight loss 24 3.0 24 5 Table 36: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Esophageal Cancer Patients Receiving Intravenous Pembrolizumab in KEYNOTE-590 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
Cisplatin
FUChemotherapy
(Cisplatin and FU)
All Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab/cisplatin/FU (range: 353 to 365 patients) and placebo/cisplatin/FU (range: 347 to 359 patients)
- † Graded per NCI CTCAE v4.03
Hematology Anemia 84 21 87 25 Neutropenia 77 44 73 41 Leukopenia 73 21 73 17 Lymphopenia 57 23 53 18 Thrombocytopenia 43 5 46 8 Chemistry Hyperglycemia 56 7 55 6 Hyponatremia 53 19 53 19 Hypoalbuminemia 53 2.8 52 2.3 Increased creatinine 45 2.5 42 2.5 Hypocalcemia 44 3.9 37 2 Hypophosphatemia 37 9 31 10 Hypokalemia 30 12 34 15 Increased alkaline phosphatase 29 1.9 29 1.7 Hyperkalemia 28 3.6 28 2.5 Increased AST 25 4.4 22 2.8 Increased ALT 23 3.6 18 1.7 Previously Treated Recurrent Locally Advanced or Metastatic Esophageal Cancer
Among the 314 patients with esophageal cancer enrolled in KEYNOTE-181 [see Clinical Studies (14.10)] treated with intravenous pembrolizumab, the median duration of exposure to intravenous pembrolizumab was 2.1 months (range: 1 day to 24.4 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Adverse reactions occurring in patients with esophageal cancer were similar to those occurring in 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent.
Cervical Cancer
FIGO 2014 Stage III-IVA Cervical Cancer with Chemoradiotherapy
The safety of intravenous pembrolizumab in combination with CRT (cisplatin plus external beam radiation therapy [EBRT] followed by brachytherapy [BT]) was investigated in KEYNOTE-A18, a placebo-controlled, randomized (1:1), multicenter, double-blind trial including 597 patients with FIGO 2014 Stage III-IVA cervical cancer [see Clinical Studies (14.11)]. Two hundred ninety-four patients received intravenous pembrolizumab in combination with chemoradiotherapy and 303 patients received placebo in combination with chemoradiotherapy.
The median duration of exposure to intravenous pembrolizumab was 20 months (range: 1 day to 32 months).
Fatal adverse reactions occurred in 1.4% of patients receiving intravenous pembrolizumab in combination with chemoradiotherapy, including 1 case each (0.3%) of large intestinal perforation, urosepsis, sepsis, and vaginal hemorrhage.
Serious adverse reactions occurred in 34% of patients receiving intravenous pembrolizumab in combination with chemoradiotherapy. Serious adverse reactions occurring in ≥1% of patients included urinary tract infection (3.1%), urosepsis (1.4%), and sepsis (1%).
Intravenous pembrolizumab was discontinued for adverse reactions in 9% of patients. The most common adverse reaction (≥1%) resulting in permanent discontinuation was diarrhea (1%).
Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 47% of patients; the most common adverse reactions leading to interruption of intravenous pembrolizumab (≥2%) were anemia (7%), COVID-19 (7%), SARS-CoV-2 test positive (4.8%), diarrhea (4.1%), increased ALT (4.1%), increased AST (3.4%) decreased neutrophil count (3.1%), and urinary tract infection (2.7%).
Table 37 and Table 38 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-A18.
Table 37: Adverse Reactions Occurring in ≥10% of Patients with FIGO 2014 Stage III-IVA Cervical Cancer Receiving Intravenous Pembrolizumab in KEYNOTE-A18 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks and 400 mg
every 6 weeks
with chemoradiotherapy
n=294Placebo
with chemoradiotherapy
n=303All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v5.0
- † Includes urinary tract infection, urinary tract infection pseudomonal, pyelonephritis acute, cystitis, Escherichia urinary tract infection
- ‡ Includes fatigue, asthenia
- § Includes hypothyroidism, autoimmune hypothyroidism
- ¶ Includes erythema multiforme, dermatitis, drug eruption, eczema, rash, skin exfoliation, dermatitis bullous, rash maculo-papular, lichen planus, dyshidrotic eczema, dermatitis acneiform
Gastrointestinal Nausea 56 0 62 2.3 Diarrhea 51 4.4 50 4.3 Vomiting 34 1.0 35 1.7 Constipation 20 0 19 0.7 Abdominal pain 13 1.0 14 1.7 Infections Urinary tract infection† 35 4.8 34 5 COVID-19 10 0 7 1.0 General Fatigue‡ 28 1.0 28 1.3 Pyrexia 14 0.7 15 0 Endocrine Hypothyroidism§ 23 0.7 8 0 Hyperthyroidism 13 0.3 3.3 0 Investigations Weight loss 19 2.4 19 1.0 Metabolism and Nutrition Decreased appetite 18 0.7 17 0.3 Renal and Urinary Dysuria 12 0.3 12 0 Skin and Subcutaneous Tissue Disorders Rash¶ 12 1.0 8 0.3 Musculoskeletal and Connective Tissues Disorders Back pain 11 0.7 11 0.7 Reproductive System Pelvic pain 11 1.0 14 1.7 Table 38: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients with FIGO 2014 Stage III-IVA Cervical Cancer Receiving Intravenous Pembrolizumab in KEYNOTE-A18 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks and
400 mg every 6 weeks
with chemoradiotherapyPlacebo
with chemoradiotherapyAll Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Laboratory abnormality percentage is based on the number of patients who had both baseline and at least one post-baseline laboratory measurement for each parameter: Intravenous pembrolizumab + chemoradiotherapy (range: 288 to 293 patients) and placebo + chemoradiotherapy (range: 299 to 301 patients)
- † Graded per NCI CTCAE v5.0
Hematology Lymphopenia 99 96 99 92 Leukopenia 96 48 94 49 Anemia 87 33 82 27 Neutropenia 76 33 76 33 Thrombocytopenia 64 9 62 7 Chemistry Hypomagnesemia 61 4.2 63 3.7 Hyponatremia 56 4.8 50 4.7 Increased AST 50 1.7 44 2.3 Increased ALT 49 3.1 46 1 Hypocalcemia 45 5 43 5 Hypokalemia 44 15 41 11 Increased creatinine 44 7 46 6 Hypoalbuminemia 38 2.4 37 2.3 Increased alkaline phosphatase 38 0.3 35 0.3 Hyperkalemia 21 2.0 16 1 Persistent, Recurrent, or Metastatic Cervical Cancer
The safety of intravenous pembrolizumab in combination with paclitaxel and cisplatin or paclitaxel and carboplatin, with or without bevacizumab, was investigated in KEYNOTE-826, a multicenter, double-blind, randomized (1:1), placebo-controlled trial in patients with persistent, recurrent, or first-line metastatic cervical cancer who had not been treated with chemotherapy except when used concurrently as a radio-sensitizing agent [see Clinical Studies (14.11)]. A total of 616 patients, regardless of tumor PD-L1 expression, received intravenous pembrolizumab 200 mg and chemotherapy with or without bevacizumab (n=307) every 3 weeks or placebo and chemotherapy with or without bevacizumab (n=309) every 3 weeks.
The median duration of exposure to intravenous pembrolizumab was 9.9 months (range: 1 day to 26 months).
Fatal adverse reactions occurred in 4.6% of patients receiving intravenous pembrolizumab in combination with chemotherapy with or without bevacizumab, including 3 cases of hemorrhage, 2 cases of sepsis, 2 cases due to unknown causes, and 1 case each of acute myocardial infarction, autoimmune encephalitis, cardiac arrest, cerebrovascular accident, femur fracture with perioperative pulmonary embolus, intestinal perforation, and pelvic infection.
Serious adverse reactions occurred in 50% of patients receiving intravenous pembrolizumab in combination with chemotherapy with or without bevacizumab. Serious adverse reactions in ≥3% of patients included febrile neutropenia (6.8%), urinary tract infection (5.2%), anemia (4.6%), acute kidney injury (3.3%), and sepsis (3.3%).
Intravenous pembrolizumab was discontinued for adverse reactions in 15% of patients. The most common adverse reaction resulting in permanent discontinuation of intravenous pembrolizumab (≥1%) was colitis (1%).
Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 66% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were thrombocytopenia (15%), neutropenia (14%), anemia (11%), increased ALT (6%), leukopenia (5%), fatigue/asthenia (4.2%), urinary tract infection (3.6%), increased AST (3.3%), pyrexia (3.3%), diarrhea (2.6%), acute kidney injury (2.6%), increased blood creatinine (2.6%), colitis (2.3%), decreased appetite (2%), and cough (2%).
For patients treated with intravenous pembrolizumab, chemotherapy, and bevacizumab (n=196), the most common (≥20%) adverse reactions were peripheral neuropathy (62%), alopecia (58%), anemia (55%), fatigue/asthenia (53%), nausea (41%), neutropenia (41%), diarrhea (39%), hypertension (35%), thrombocytopenia (35%), constipation (31%), arthralgia (31%), vomiting (30%), urinary tract infection (27%), rash (26%), leukopenia (24%), hypothyroidism (22%), and decreased appetite (21%).
Table 39 and Table 40 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-826.
Table 39: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-826 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
and chemotherapy* with or without bevacizumab
n=307Placebo
and chemotherapy* with or without bevacizumab
n=309All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin)
- † Graded per NCI CTCAE v4.0
- ‡ Includes neuropathy peripheral, peripheral sensory neuropathy, peripheral motor neuropathy, peripheral sensorimotor neuropathy, paresthesia
- § Includes rash, rash maculo-papular, rash erythematous, rash macular, rash papular, rash pruritic, rash pustular
- ¶ Includes fatigue, asthenia
Nervous System Peripheral neuropathy‡ 58 4.2 57 6 Skin and Subcutaneous Tissue Alopecia 56 0 58 0 Rash§ 22 3.6 15 0.3 General Fatigue¶ 47 7 46 6 Gastrointestinal Nausea 40 2 44 1.6 Diarrhea 36 2 30 2.6 Constipation 28 0.3 33 1 Vomiting 26 2.6 27 1.9 Musculoskeletal and Connective Tissue Arthralgia 27 0.7 26 1.3 Vascular Hypertension 24 9 23 11 Infections Urinary tract infection 24 9 26 8 Table 40: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-826 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
and chemotherapy† with or without bevacizumab
n=307Placebo
and chemotherapy† with or without bevacizumab
n=309All Grades‡
(%)Grades 3-4
(%)All Grades‡
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab plus chemotherapy (range: 296 to 301 patients) and placebo plus chemotherapy (range: 299 to 302 patients)
- † Chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin)
- ‡ Graded per NCI CTCAE v4.0
Hematology Anemia 80 35 77 33 Leukopenia 76 27 69 19 Neutropenia 73 43 62 32 Lymphopenia 64 35 59 35 Thrombocytopenia 57 19 53 15 Chemistry Hyperglycemia 51 4.7 46 2.3 Hypoalbuminemia 46 1.4 37 5 Hyponatremia 39 14 38 11 Increased ALT 40 7 38 6 Increased AST 40 6 36 3.0 Increased alkaline phosphatase 38 3.4 40 2.3 Hypocalcemia 37 4.1 31 5 Increased creatinine 34 5 32 6 Hypokalemia 29 7 26 7 Hyperkalemia 23 3.7 27 4.7 Hypercalcemia 21 1.0 20 1.3 Previously Treated Recurrent or Metastatic Cervical Cancer
Among the 98 patients with cervical cancer enrolled in Cohort E of KEYNOTE-158 [see Clinical Studies (14.11)], the median duration of exposure to intravenous pembrolizumab was 2.9 months (range: 1 day to 22.1 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible.
Intravenous pembrolizumab was discontinued due to adverse reactions in 8% of patients. Serious adverse reactions occurred in 39% of patients receiving intravenous pembrolizumab. The most frequent serious adverse reactions reported included anemia (7%), fistula (4.1%), hemorrhage (4.1%), and infections [except UTIs] (4.1%). Tables 41 and 42 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-158.
Table 41: Adverse Reactions Occurring in ≥10% of Patients with Cervical Cancer in KEYNOTE-158 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
N=98All Grades*
(%)Grades 3–4
(%)- * Graded per NCI CTCAE v4.0
- † Includes asthenia, fatigue, lethargy, malaise
- ‡ Includes breast pain, cancer pain, dysesthesia, dysuria, ear pain, gingival pain, groin pain, lymph node pain, oropharyngeal pain, pain, pain of skin, pelvic pain, radicular pain, stoma site pain, toothache
- § Includes edema peripheral, peripheral swelling
- ¶ Includes arthralgia, back pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, myositis, neck pain, non-cardiac chest pain, pain in extremity
- # Includes colitis, diarrhea, gastroenteritis
- Þ Includes abdominal discomfort, abdominal distension, abdominal pain, abdominal pain lower, abdominal pain upper
- ß Includes epistaxis, hematuria, hemoptysis, metrorrhagia, rectal hemorrhage, uterine hemorrhage, vaginal hemorrhage
- à Includes bacterial pyelonephritis, pyelonephritis acute, urinary tract infection, urinary tract infection bacterial, urinary tract infection pseudomonal, urosepsis
- è Includes cellulitis, clostridium difficile infection, device-related infection, empyema, erysipelas, herpes virus infection, infected neoplasm, infection, influenza, lower respiratory tract congestion, lung infection, oral candidiasis, oral fungal infection, osteomyelitis, pseudomonas infection, respiratory tract infection, tooth abscess, upper respiratory tract infection, uterine abscess, vulvovaginal candidiasis
- ð Includes dermatitis, drug eruption, eczema, erythema, palmar-plantar erythrodysesthesia syndrome, rash, rash generalized, rash maculo-papular
General Fatigue† 43 5 Pain‡ 22 2.0 Pyrexia 19 1.0 Edema peripheral§ 15 2.0 Musculoskeletal and Connective Tissue Musculoskeletal pain¶ 27 5 Gastrointestinal Diarrhea# 23 2.0 Abdominal painÞ 22 3.1 Nausea 19 0 Vomiting 19 1.0 Constipation 14 0 Metabolism and Nutrition Decreased appetite 21 0 Vascular Hemorrhageß 19 5 Infections UTIà 18 6 Infection (except UTI)è 16 4.1 Skin and Subcutaneous Tissue Rashð 17 2.0 Endocrine Hypothyroidism 11 0 Nervous System Headache 11 2.0 Respiratory, Thoracic and Mediastinal Dyspnea 10 1.0 Table 42: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients with Cervical Cancer in KEYNOTE-158 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeksAll Grades†
(%)Grades 3-4
(%)- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 76 to 79 patients)
- † Graded per NCI CTCAE v4.0
Hematology Anemia 54 24 Lymphopenia 45 9 Chemistry Hypoalbuminemia 44 5 Increased alkaline phosphatase 40 1.3 Hyponatremia 38 13 Hyperglycemia 38 1.3 Increased AST 34 3.9 Increased creatinine 32 5 Hypocalcemia 27 0 Increased ALT 21 3.9 Hypokalemia 20 6 Other laboratory abnormalities occurring in ≥10% of patients receiving intravenous pembrolizumab were hypophosphatemia (19% all Grades; 6% Grades 3-4), increased INR (17% all Grades; 0% Grades 3-4), hypercalcemia (14% all Grades; 2.6% Grades 3-4), platelet count decreased (14% all Grades; 1.3% Grades 3-4), activated partial thromboplastin time prolonged (10% all Grades; 0% Grades 3-4), hypoglycemia (13% all Grades; 1.3% Grades 3-4), white blood cell decreased (13% all Grades; 2.6% Grades 3-4), and hyperkalemia (13% all Grades; 1.3% Grades 3-4).
HCC
Previously Treated HCC
The safety of intravenous pembrolizumab was investigated in KEYNOTE-394, a multicenter, double-blind, randomized, placebo-controlled trial that enrolled patients with previously treated HCC. Patients were randomized (2:1) and received intravenous pembrolizumab 200 mg (n=299) or placebo (n=153) intravenously every 3 weeks for up to 35 cycles [see Clinical Studies (14.12)].
The median duration of exposure was 3.3 months (range: 1 day to 27.3 months) in the intravenous pembrolizumab arm and 2.2 months (range: 1 day to 15.5 months) in the placebo arm. Intravenous pembrolizumab was discontinued due to adverse reactions in 13% of patients. The most common adverse reaction resulting in permanent discontinuation of intravenous pembrolizumab was ascites (2.3%). Adverse reactions leading to interruption of intravenous pembrolizumab occurred in 26% of patients; the most common adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were increased blood bilirubin (9%), increased AST (5%), and increased ALT (2%).
Tables 43 and 44 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-394.
Table 43: Adverse Reactions Occurring in ≥10% of Patients with HCC Receiving Intravenous Pembrolizumab in KEYNOTE-394 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
n=299Placebo
n=153All Grades*
(%)Grades 3-5
(%)All Grades*
(%)Grades 3-5
(%)- * Graded per NCI CTCAE v4.03
- † Includes dermatitis, dermatitis allergic, dermatitis bullous, rash, rash erythematous, rash maculo-papular, rash pustular, and blister.
General Pyrexia 18 0.7 14 0 Skin and Subcutaneous Tissue Rash† 18 0.7 7 0 Pruritus 12 0 4 0 Gastrointestinal Diarrhea 16 1.7 9 0 Metabolism and Nutrition Decreased appetite 15 0.3 9 0 Infections Upper respiratory tract infection 11 1.0 7 0.7 Respiratory, Thoracic, and Mediastinal Cough 11 0 9 0 Endocrine Hypothyroidism 10 0 7 0 Table 44: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients with HCC Receiving Intravenous Pembrolizumab in KEYNOTE-394 Laboratory Test* Intravenous Pembrolizumab Placebo All Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 223 to 297 patients) and placebo (range: 144 to 151 patients).
- † Graded per NCI CTCAE v4.03
Chemistry Increased AST 54 14 44 12 Increased bilirubin 47 11 36 7 Increased ALT 47 7 32 4.6 Increased gamma-glutamyl transferase (GGT) 40 20 39 15 Hypoalbuminemia 40 0.7 20 0.7 Increased alkaline phosphatase 39 4.1 34 4 Hyperglycemia 36 3.3 26 1.4 Hyponatremia 36 11 28 5 Hypophosphatemia 30 6 17 4 Hypocalcemia 24 1.4 15 0.7 Hematology Lymphopenia 44 11 34 4.6 Anemia 36 7 30 3.3 Decreased platelets 32 4.7 29 2 Leukopenia 30 1.3 21 0.7 Neutropenia 25 4.4 21 2 BTC
The safety of intravenous pembrolizumab in combination with gemcitabine and cisplatin, was investigated in KEYNOTE-966, a multicenter, double-blind, randomized, placebo-controlled trial in patients with locally advanced unresectable or metastatic BTC who had not received prior systemic therapy in the advanced disease setting [see Clinical Studies (14.13)]. A total of 1063 patients received either intravenous pembrolizumab 200 mg plus gemcitabine and cisplatin chemotherapy (n=529) or placebo plus gemcitabine and cisplatin chemotherapy (n=534) every 3 weeks.
The median duration of exposure to intravenous pembrolizumab was 6 months (range: 1 day to 28 months).
Intravenous pembrolizumab was discontinued for adverse reactions in 15% of patients. The most common adverse reaction resulting in permanent discontinuation of intravenous pembrolizumab (≥1%) was pneumonitis (1.3%).
Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 55% of patients. The most common adverse reactions or laboratory abnormalities leading to interruption of intravenous pembrolizumab (≥2%) were decreased neutrophil count (18%), decreased platelet count (10%), anemia (6%), decreased white blood count (4%), pyrexia (3.8%), fatigue (3.0%), cholangitis (2.8%), increased ALT (2.6%), increased AST (2.5%), and biliary obstruction (2.3%).
In the intravenous pembrolizumab plus chemotherapy versus placebo plus chemotherapy arms, there was a difference of ≥5% incidence in adverse reactions between patients treated with intravenous pembrolizumab versus placebo for pyrexia (26% vs 20%), rash (21% vs 13%), pruritus (15% vs 10%), and hypothyroidism (9% vs. 2.6%). There were no clinically meaningful differences in incidence of Grade 3-4 toxicity between arms.
There was a difference of ≥5% incidence in laboratory abnormalities between patients treated with intravenous pembrolizumab plus chemotherapy versus placebo plus chemotherapy for decreased lymphocytes (69% vs 61%). There were no clinically meaningful differences in incidence of Grade 3-4 toxicity between arms.
MCC
Among the 105 patients with MCC enrolled in KEYNOTE-017 and KEYNOTE-913 [see Clinical Studies (14.14)], the median duration of exposure to intravenous pembrolizumab was 6.3 months (range 1 day to 28 months). Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Adverse reactions occurring in patients with MCC were similar to those occurring in 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent. Laboratory abnormalities (Grades 3-4) that occurred at a higher incidence included increased lipase (17%).
RCC
In combination with axitinib in the first-line treatment of advanced RCC (KEYNOTE-426)
The safety of intravenous pembrolizumab in combination with axitinib was investigated in KEYNOTE-426 [see Clinical Studies (14.15)]. Patients with medical conditions that required systemic corticosteroids or other immunosuppressive medications or had a history of severe autoimmune disease other than type 1 diabetes, vitiligo, Sjogren's syndrome, and hypothyroidism stable on hormone replacement were ineligible. Patients received intravenous pembrolizumab 200 mg intravenously every 3 weeks and axitinib 5 mg orally twice daily, or sunitinib 50 mg once daily for 4 weeks and then off treatment for 2 weeks. The median duration of exposure to the combination therapy of intravenous pembrolizumab and axitinib was 10.4 months (range: 1 day to 21.2 months).
The study population characteristics were: median age of 62 years (range: 30 to 89), 40% age 65 or older; 71% male; 80% White; and 80% Karnofsky Performance Status (KPS) of 90-100 and 20% KPS of 70-80.
Fatal adverse reactions occurred in 3.3% of patients receiving intravenous pembrolizumab in combination with axitinib. These included 3 cases of cardiac arrest, 2 cases of pulmonary embolism and 1 case each of cardiac failure, death due to unknown cause, myasthenia gravis, myocarditis, Fournier's gangrene, plasma cell myeloma, pleural effusion, pneumonitis, and respiratory failure.
Serious adverse reactions occurred in 40% of patients receiving intravenous pembrolizumab in combination with axitinib. Serious adverse reactions in ≥1% of patients receiving intravenous pembrolizumab in combination with axitinib included hepatotoxicity (7%), diarrhea (4.2%), acute kidney injury (2.3%), dehydration (1%), and pneumonitis (1%).
Permanent discontinuation due to an adverse reaction of either intravenous pembrolizumab or axitinib occurred in 31% of patients; 13% intravenous pembrolizumab only, 13% axitinib only, and 8% both drugs. The most common adverse reaction (>1%) resulting in permanent discontinuation of intravenous pembrolizumab, axitinib, or the combination was hepatotoxicity (13%), diarrhea/colitis (1.9%), acute kidney injury (1.6%), and cerebrovascular accident (1.2%).
Dose interruptions or reductions due to an adverse reaction, excluding temporary interruptions of intravenous pembrolizumab infusions due to infusion-related reactions, occurred in 76% of patients receiving intravenous pembrolizumab in combination with axitinib. This includes interruption of intravenous pembrolizumab in 50% of patients. Axitinib was interrupted in 64% of patients and dose reduced in 22% of patients. The most common adverse reactions (>10%) resulting in interruption of intravenous pembrolizumab were hepatotoxicity (14%) and diarrhea (11%), and the most common adverse reactions (>10%) resulting in either interruption or reduction of axitinib were hepatotoxicity (21%), diarrhea (19%), and hypertension (18%).
The most common adverse reactions (≥20%) in patients receiving intravenous pembrolizumab and axitinib were diarrhea, fatigue/asthenia, hypertension, hypothyroidism, decreased appetite, hepatotoxicity, palmar-plantar erythrodysesthesia, nausea, stomatitis/mucosal inflammation, dysphonia, rash, cough, and constipation.
Twenty-seven percent (27%) of patients treated with intravenous pembrolizumab in combination with axitinib received an oral prednisone dose equivalent to ≥40 mg daily for an immune-mediated adverse reaction.
Tables 45 and 46 summarize the adverse reactions and laboratory abnormalities, respectively, that occurred in at least 20% of patients treated with intravenous pembrolizumab and axitinib in KEYNOTE-426.
Table 45: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab with Axitinib in KEYNOTE-426 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
and Axitinib
n=429Sunitinib
n=425
All Grades*
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes diarrhea, colitis, enterocolitis, gastroenteritis, enteritis, enterocolitis hemorrhagic
- ‡ Includes hypertension, blood pressure increased, hypertensive crisis, labile hypertension
- § Includes ALT increased, AST increased, autoimmune hepatitis, blood bilirubin increased, drug-induced liver injury, hepatic enzyme increased, hepatic function abnormal, hepatitis, hepatitis fulminant, hepatocellular injury, hepatotoxicity, hyperbilirubinemia, immune-mediated hepatitis, liver function test increased, liver injury, transaminases increased
- ¶ Includes rash, butterfly rash, dermatitis, dermatitis acneform, dermatitis atopic, dermatitis bullous, dermatitis contact, exfoliative rash, genital rash, rash erythematous, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, seborrheic dermatitis, skin discoloration, skin exfoliation, perineal rash
Gastrointestinal Diarrhea† 56 11 45 5 Nausea 28 0.9 32 0.9 Constipation 21 0 15 0.2 General Fatigue/Asthenia 52 5 51 10 Vascular Hypertension‡ 48 24 48 20 Hepatobiliary Hepatotoxicity§ 39 20 25 4.9 Endocrine Hypothyroidism 35 0.2 32 0.2 Metabolism and Nutrition Decreased appetite 30 2.8 29 0.7 Skin and Subcutaneous Tissue Palmar-plantar erythrodysesthesia syndrome 28 5 40 3.8 Stomatitis/Mucosal inflammation 27 1.6 41 4 Rash¶ 25 1.4 21 0.7 Respiratory, Thoracic and Mediastinal Dysphonia 25 0.2 3.3 0 Cough 21 0.2 14 0.5 Table 46: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab with Axitinib in KEYNOTE-426 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
and AxitinibSunitinib All Grades†
%Grades 3-4
%All Grades
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab/axitinib (range: 342 to 425 patients) and sunitinib (range: 345 to 421 patients).
- † Graded per NCI CTCAE v4.03
- ‡ Corrected for albumin
- § Two patients with a Grade 3 elevated activated partial thromboplastin time prolonged (aPTT) were also reported as having an adverse reaction of hepatotoxicity.
Chemistry Hyperglycemia 62 9 54 3.2 Increased ALT 60 20 44 5 Increased AST 57 13 56 5 Increased creatinine 43 4.3 40 2.4 Hyponatremia 35 8 29 8 Hyperkalemia 34 6 22 1.7 Hypoalbuminemia 32 0.5 34 1.7 Hypercalcemia 27 0.7 15 1.9 Hypophosphatemia 26 6 49 17 Increased alkaline phosphatase 26 1.7 30 2.7 Hypocalcemia‡ 22 0.2 29 0.7 Blood bilirubin increased 22 2.1 21 1.9 Activated partial thromboplastin time prolonged§ 22 1.2 14 0 Hematology Lymphopenia 33 11 47 9 Anemia 29 2.1 65 8 Thrombocytopenia 27 1.4 78 14 In combination with lenvatinib in the first-line treatment of advanced RCC (KEYNOTE-581)
The safety of intravenous pembrolizumab was evaluated in KEYNOTE-581 [see Clinical Studies (14.15)]. Patients received intravenous pembrolizumab 200 mg intravenously every 3 weeks in combination with lenvatinib 20 mg orally once daily (n=352), or lenvatinib 18 mg orally once daily in combination with everolimus 5 mg orally once daily (n=355), or sunitinib 50 mg orally once daily for 4 weeks then off treatment for 2 weeks (n=340). The median duration of exposure to the combination therapy of intravenous pembrolizumab and lenvatinib was 17 months (range: 0.1 to 39).
Fatal adverse reactions occurred in 4.3% of patients treated with intravenous pembrolizumab in combination with lenvatinib, including cardio-respiratory arrest (0.9%), sepsis (0.9%), and one case (0.3%) each of arrhythmia, autoimmune hepatitis, dyspnea, hypertensive crisis, increased blood creatinine, multiple organ dysfunction syndrome, myasthenic syndrome, myocarditis, nephritis, pneumonitis, ruptured aneurysm, and subarachnoid hemorrhage.
Serious adverse reactions occurred in 51% of patients receiving intravenous pembrolizumab and lenvatinib. Serious adverse reactions in ≥2% of patients were hemorrhagic events (5%), diarrhea (4%), hypertension (3%), myocardial infarction (3%), pneumonitis (3%), vomiting (3%), acute kidney injury (2%), adrenal insufficiency (2%), dyspnea (2%), and pneumonia (2%).
Permanent discontinuation of either of intravenous pembrolizumab, lenvatinib or both due to an adverse reaction occurred in 37% of patients receiving intravenous pembrolizumab in combination with lenvatinib; 29% intravenous pembrolizumab only, 26% lenvatinib only, and 13% both. The most common adverse reactions (≥2%) resulting in permanent discontinuation of intravenous pembrolizumab, lenvatinib, or the combination were pneumonitis (3%), myocardial infarction (3%), hepatotoxicity (3%), acute kidney injury (3%), rash (3%), and diarrhea (2%).
Dose interruptions of intravenous pembrolizumab, lenvatinib, or both due to an adverse reaction occurred in 78% of patients receiving intravenous pembrolizumab in combination with lenvatinib. Intravenous pembrolizumab was interrupted in 55% of patients and both drugs were interrupted in 39% of patients. The most common adverse reactions (≥3%) resulting in interruption of intravenous pembrolizumab were diarrhea (10%), hepatotoxicity (8%), fatigue (7%), lipase increased (5%), amylase increased (4%), musculoskeletal pain (3%), hypertension (3%), rash (3%), acute kidney injury (3%), and decreased appetite (3%).
Fifteen percent (15%) of patients treated with intravenous pembrolizumab in combination with lenvatinib received an oral prednisone equivalent to ≥40 mg daily for an immune-mediated adverse reaction.
Tables 47 and 48 summarize the adverse reactions and laboratory abnormalities, respectively, that occurred in ≥20% of patients treated with intravenous pembrolizumab and lenvatinib in KEYNOTE-581.
Table 47: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab with Lenvatinib in KEYNOTE-581 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
with Lenvatinib
N=352Sunitinib 50 mg
N=340All Grades
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Includes asthenia, fatigue, lethargy, malaise
- † Includes diarrhea, gastroenteritis
- ‡ Includes aphthous ulcer, gingival pain, glossitis, glossodynia, mouth ulceration, mucosal inflammation, oral discomfort, oral mucosal blistering, oral pain, oropharyngeal pain, pharyngeal inflammation, stomatitis
- § Includes abdominal discomfort, abdominal pain, abdominal rigidity, abdominal tenderness, epigastric discomfort, lower abdominal pain, upper abdominal pain
- ¶ Includes arthralgia, arthritis, back pain, bone pain, breast pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, non-cardiac chest pain, pain in extremity, pain in jaw
- # Includes hypothyroidism, increased blood thyroid stimulating hormone, secondary hypothyroidism
- Þ Includes essential hypertension, increased blood pressure, increased diastolic blood pressure, hypertension, hypertensive crisis, hypertensive retinopathy, labile blood pressure
- ß Includes all hemorrhage terms. Hemorrhage terms that occurred in 1 or more subjects in either treatment group include Anal hemorrhage, aneurysm ruptured, blood blister, blood loss anemia, blood urine present, catheter site hematoma, cerebral microhemorrhage, conjunctival hemorrhage, contusion, diarrhea hemorrhagic, disseminated intravascular coagulation, ecchymosis, epistaxis, eye hemorrhage, gastric hemorrhage, gastritis hemorrhagic, gingival bleeding, hemorrhage urinary tract, hemothorax, hematemesis, hematoma, hematochezia, hematuria, hemoptysis, hemorrhoidal hemorrhage, increased tendency to bruise, injection site hematoma, injection site hemorrhage, intra-abdominal hemorrhage, lower gastrointestinal hemorrhage, Mallory-Weiss syndrome, melaena, petechiae, rectal hemorrhage, renal hemorrhage, retroperitoneal hemorrhage, small intestinal hemorrhage, splinter hemorrhages, subcutaneous hematoma, subdural hematoma, subarachnoid hemorrhage, thrombotic thrombocytopenic purpura, tumor hemorrhage, traumatic hematoma, upper gastrointestinal hemorrhage
- à Includes decreased appetite, early satiety
- è Includes genital rash, infusion site rash, penile rash, perineal rash, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic, rash pustular
- ð Includes palmar erythema, palmar-plantar erythrodysesthesia syndrome, plantar erythema
- ø Includes hemoglobinuria, nephrotic syndrome, proteinuria
- ý Includes acute kidney injury, azotemia, blood creatinine increased, creatinine renal clearance decreased, hypercreatininemia, renal failure, renal impairment, oliguria, glomerular filtration rate decreased, and nephropathy toxic
- £ Includes alanine aminotransferase increased, aspartate aminotransferase increased, blood bilirubin increased, drug-induced liver injury, hepatic enzyme increased, hepatic failure, hepatic function abnormal, hepatocellular injury, hepatotoxicity, hyperbilirubinemia, hypertransaminasemia, immune-mediated hepatitis, liver function test increased, liver injury, transaminases increased, gamma-glutamyltransferase increased
General Fatigue* 63 9 56 8 Gastrointestinal Diarrhea† 62 10 50 6 Stomatitis‡ 43 2 43 2 Nausea 36 3 33 1 Abdominal pain§ 27 2 18 1 Vomiting 26 3 20 1 Constipation 25 1 19 0 Musculoskeletal and Connective Tissue Musculoskeletal disorders¶ 58 4 41 3 Endocrine Hypothyroidism# 57 1 32 0 Vascular HypertensionÞ 56 29 43 20 Hemorrhagic eventsß 27 5 26 4 Metabolism Decreased appetiteà 41 4 31 1 Skin and Subcutaneous Tissue Rashè 37 5 17 1 Palmar-plantar erythrodysesthesia syndromeð 29 4 38 4 Investigations Weight loss 30 8 9 0.3 Respiratory, Thoracic and Mediastinal Dysphonia 30 0 4 0 Renal and Urinary Proteinuriaø 30 8 13 3 Acute kidney injuryý 21 5 16 2 Hepatobiliary Hepatotoxicity£ 25 9 21 5 Nervous System Headache 23 1 16 1 Clinically relevant adverse reactions (<20%) that occurred in patients receiving intravenous pembrolizumab with lenvatinib were myocardial infarction (3%) and angina pectoris (1%).
Table 48: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% (All Grades) of Patients Receiving Intravenous Pembrolizumab with Lenvatinib in KEYNOTE-581 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
with LenvatinibSunitinib 50 mg All Grades
%†Grade 3-4
%†All Grades
%†Grade 3-4
%†- * With at least one Grade increase from baseline
- † Laboratory abnormality percentage is based on the number of patients who had both baseline and at least one post-baseline laboratory measurement for each parameter: intravenous pembrolizumab with lenvatinib (range: 343 to 349 patients) and sunitinib (range: 329 to 335 patients).
Chemistry Hypertriglyceridemia 80 15 71 15 Hypercholesterolemia 64 5 43 1 Increased lipase 61 34 59 28 Increased creatinine 61 5 61 2 Increased amylase 59 17 41 9 Increased AST 58 7 57 3 Hyperglycemia 55 7 48 3 Increased ALT 52 7 49 4 Hyperkalemia 44 9 28 6 Hypoglycemia 44 2 27 1 Hyponatremia 41 12 28 9 Decreased albumin 34 0.3 22 0 Increased alkaline phosphatase 32 4 32 1 Hypocalcemia 30 2 22 1 Hypophosphatemia 29 7 50 8 Hypomagnesemia 25 2 15 3 Increased creatine phosphokinase 24 6 36 5 Hypermagnesemia 23 2 22 3 Hypercalcemia 21 1 11 1 Hematology Lymphopenia 54 9 66 15 Thrombocytopenia 39 2 73 13 Anemia 38 3 66 8 Leukopenia 34 1 77 8 Neutropenia 31 4 72 16 Grade 3 and 4 increased ALT or AST was seen in 9% of patients. Grade ≥2 increased ALT or AST was reported in 64 (18%) patients, of whom 20 (31%) received ≥40 mg daily oral prednisone equivalent. Recurrence of Grade ≥2 increased ALT or AST was observed on rechallenge in 10 patients receiving both intravenous pembrolizumab and lenvatinib (n=38) and was not observed on rechallenge with intravenous pembrolizumab alone (n=3).
Adjuvant treatment of RCC
The safety of intravenous pembrolizumab as a single agent was investigated in KEYNOTE-564, a randomized (1:1) double-blind placebo-controlled trial in which 984 patients who had undergone nephrectomy for RCC received 200 mg of intravenous pembrolizumab by intravenous infusion every 3 weeks (n=488) or placebo (n=496) for up to one year [see Clinical Studies (14.15)]. The median duration of exposure to intravenous pembrolizumab was 11.1 months (range: 1 day to 14.3 months). Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible.
Serious adverse reactions occurred in 20% of these patients receiving intravenous pembrolizumab. Serious adverse reactions (≥1%) were acute kidney injury, adrenal insufficiency, pneumonia, colitis, and diabetic ketoacidosis (1% each). Fatal adverse reactions occurred in 0.2% of those treated with intravenous pembrolizumab, including one case of pneumonia.
Discontinuation of intravenous pembrolizumab due to an adverse reaction occurred in 21% of patients; the most common (≥1%) were increased ALT (1.6%), colitis (1%), and adrenal insufficiency (1%).
Dose interruptions of intravenous pembrolizumab due to an adverse reaction occurred in 26% of patients; the most common (≥1%) were increased AST (2.3%), arthralgia (1.6%), hypothyroidism (1.6%), diarrhea (1.4%), increased ALT (1.4%), fatigue (1.4%), rash, decreased appetite, and vomiting (1% each).
Tables 49 and 50 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in KEYNOTE-564.
Table 49: Selected* Adverse Reactions Occurring in ≥10% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-564 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
n=488Placebo
n=496All Grades†
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)- * Adverse reactions occurring at same or higher incidence than in placebo arm
- † Graded per NCI CTCAE v4.0
- ‡ Includes arthralgia, back pain, myalgia, arthritis, pain in extremity, neck pain, musculoskeletal pain, musculoskeletal stiffness, spinal pain, musculoskeletal chest pain, bone pain, musculoskeletal discomfort
- § Includes asthenia, fatigue
- ¶ Includes rash, rash maculo-papular, rash papular, skin exfoliation, lichen planus, rash erythematous, eczema, rash macular, dermatitis acneiform, dermatitis, rash pruritic, Stevens-Johnson Syndrome, eczema asteatotic, palmar-plantar erythrodysesthesia syndrome
- # Includes diarrhea, colitis, enterocolitis, frequent bowel movements, enteritis
- Þ Includes abdominal pain, abdominal pain lower, abdominal pain upper, abdominal discomfort, gastrointestinal pain
- ß Includes upper-airway cough syndrome, productive cough, cough
- à Includes tension headache, headache, sinus headache, migraine with aura
- è Includes alanine aminotransferase increased, aspartate aminotransferase increased, blood bilirubin increased, drug-induced liver injury, hepatic enzyme increased, hepatic function abnormal, hepatocellular injury, hepatotoxicity, hyperbilirubinemia, immune-mediated hepatitis, liver function test increased, transaminases increased, gamma-glutamyltransferase increased, bilirubin conjugated increased
- ð Includes acute kidney injury, blood creatinine increased, renal failure, renal impairment, oliguria, glomerular filtration rate decreased, nephropathy toxic
Musculoskeletal and Connective Tissue Musculoskeletal pain‡ 41 1.2 36 0.6 General Fatigue§ 40 1.2 31 0.2 Skin and Subcutaneous Tissue Rash¶ 30 1.4 15 0.4 Pruritus 23 0.2 13 0 Gastrointestinal Diarrhea# 27 2.7 23 0.2 Nausea 16 0.4 10 0 Abdominal painÞ 11 0.4 13 0.2 Endocrine Hypothyroidism 21 0.2 3.6 0 Hyperthyroidism 12 0.2 0.2 0 Respiratory, Thoracic and Mediastinal Cough ß 17 0 12 0 Nervous System Headacheà 15 0.2 13 0 Hepatobiliary Hepatotoxicityè 14 3.7 7 0.6 Renal and Urinary Acute kidney injuryð 13 1.2 10 0.2 Table 50: Selected* Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-564 Laboratory Test† Intravenous Pembrolizumab
200 mg every 3 weeksPlacebo All Grades‡
%Grades 3-4
%All Grades
%Grades 3-4
%- * Laboratory abnormalities occurring at same or higher incidence than placebo
- † Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab (range: 440 to 449 patients) and placebo (range: 461 to 469 patients); increased INR: intravenous pembrolizumab n=199 and placebo n=224.
- ‡ Graded per NCI CTCAE v4.03
Chemistry Hyperglycemia 48 8 45 4.5 Increased creatinine 39 1.1 28 0.2 Increased INR 29 1.0 20 0.9 Hyponatremia 21 3.3 13 1.9 Increased ALT 20 3.6 11 0.2 Hematology Anemia 28 0.5 20 0.4 Endometrial Carcinoma
Primary Advanced or Recurrent Endometrial Carcinoma
The safety of intravenous pembrolizumab in combination with chemotherapy (paclitaxel and carboplatin) was investigated in KEYNOTE-868, a randomized (1:1), multicenter, double-blind, placebo-controlled trial that enrolled patients with advanced or recurrent endometrial carcinoma [see Clinical Studies (14.16)]. A total of 759 patients received intravenous pembrolizumab 200 mg every 3 weeks and chemotherapy for 6 cycles followed by intravenous pembrolizumab 400 mg every 6 weeks for up to 14 cycles (n=382) or placebo and chemotherapy for 6 cycles followed by placebo for up to 14 cycles (n=377). The median duration of exposure to intravenous pembrolizumab was 5.6 months (range: 1 day to 24.0 months).
Serious adverse reactions occurred in 35% of patients receiving intravenous pembrolizumab in combination with chemotherapy, compared to 19% of patients receiving placebo in combination with chemotherapy.
Fatal adverse reactions occurred in 1.6% of patients receiving intravenous pembrolizumab in combination with chemotherapy, including COVID-19 (0.5%), and cardiac arrest (0.3%).
Intravenous pembrolizumab was discontinued for an adverse reaction in 14% of patients. Chemotherapy dose reduction was required in 29% of patients receiving intravenous pembrolizumab in combination with chemotherapy, compared to 23% of patients receiving placebo in combination with chemotherapy. There were no clinically meaningful differences in chemotherapy discontinuations or interruptions between arms.
Adverse reactions occurring in patients treated with intravenous pembrolizumab and chemotherapy were generally similar to those observed with intravenous pembrolizumab alone or chemotherapy alone with the exception of rash (33% all Grades; 2.9% Grades 3-4).
In Combination with Lenvatinib for the Treatment of Advanced Endometrial Carcinoma That Is pMMR or Not MSI-H.
The safety of intravenous pembrolizumab in combination with lenvatinib was investigated in KEYNOTE-775, a multicenter, open-label, randomized (1:1), active-controlled trial in patients with advanced endometrial carcinoma previously treated with at least one prior platinum-based chemotherapy regimen in any setting, including in the neoadjuvant and adjuvant settings [see Clinical Studies (14.16)]. Patients with endometrial carcinoma that is pMMR or not MSI-H received intravenous pembrolizumab 200 mg every 3 weeks in combination with lenvatinib 20mg orally once daily (n=342) or received doxorubicin or paclitaxel (n=325).
For patients with pMMR or not MSI-H tumor status, the median duration of study treatment was 7.2 months (range 1 day to 26.8 months) and the median duration of exposure to intravenous pembrolizumab was 6.8 months (range: 1 day to 25.8 months).
Fatal adverse reactions among these patients occurred in 4.7% of those treated with intravenous pembrolizumab and lenvatinib, including 2 cases of pneumonia, and 1 case of the following: acute kidney injury, acute myocardial infarction, colitis, decreased appetite, intestinal perforation, lower gastrointestinal hemorrhage, malignant gastrointestinal obstruction, multiple organ dysfunction syndrome, myelodysplastic syndrome, pulmonary embolism, and right ventricular dysfunction.
Serious adverse reactions occurred in 50% of these patients receiving intravenous pembrolizumab and lenvatinib. Serious adverse reactions (≥3%) were hypertension (4.4%) and urinary tract infections (3.2%).
Discontinuation of intravenous pembrolizumab due to an adverse reaction occurred in 15% of these patients. The most common adverse reaction leading to discontinuation of intravenous pembrolizumab (≥1%) was increased ALT (1.2%).
Dose interruptions of intravenous pembrolizumab due to an adverse reaction occurred in 48% of these patients. The most common adverse reactions leading to interruption of intravenous pembrolizumab (≥3%) were diarrhea (8%), increased ALT (4.4%), increased AST (3.8%), and hypertension (3.5%).
Tables 51 and 52 summarize adverse reactions and laboratory abnormalities, respectively, in patients on intravenous pembrolizumab in combination with lenvatinib in KEYNOTE-775.
Table 51: Adverse Reactions Occurring in ≥20% of Patients with Endometrial Carcinoma in KEYNOTE-775 Endometrial Carcinoma (pMMR or not MSI-H) Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
and Lenvatinib
n=342Doxorubicin or
Paclitaxel
n=325All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes hypothyroidism, blood thyroid stimulating hormone increased, thyroiditis, secondary hypothyroidism
- ‡ Includes hypertension, blood pressure increased, secondary hypertension, blood pressure abnormal, hypertensive encephalopathy, blood pressure fluctuation
- § Includes epistaxis, vaginal hemorrhage, hematuria, gingival bleeding, metrorrhagia, rectal hemorrhage, contusion, hematochezia, cerebral hemorrhage, conjunctival hemorrhage, gastrointestinal hemorrhage, hemoptysis, hemorrhage urinary tract, lower gastrointestinal hemorrhage, mouth hemorrhage, petechiae, uterine hemorrhage, anal hemorrhage, blood blister, eye hemorrhage, hematoma, hemorrhage intracranial, hemorrhagic stroke, melena, stoma site hemorrhage, upper gastrointestinal hemorrhage, wound hemorrhage, blood urine present, ecchymosis, hematemesis, hemorrhage subcutaneous, hepatic hematoma, injection site bruising, intestinal hemorrhage, laryngeal hemorrhage, pulmonary hemorrhage, subdural hematoma, umbilical hemorrhage, vessel puncture site bruise
- ¶ Includes fatigue, asthenia, malaise, lethargy
- # Includes diarrhea, gastroenteritis
- Þ Includes stomatitis, mucosal inflammation, oropharyngeal pain, aphthous ulcer, mouth ulceration, cheilitis, oral mucosal erythema, tongue ulceration
- ß Includes abdominal pain, abdominal pain upper, abdominal pain lower, abdominal discomfort, gastrointestinal pain, abdominal tenderness, epigastric discomfort
- à Includes arthralgia, myalgia, back pain, pain in extremity, bone pain, neck pain, musculoskeletal pain, arthritis, musculoskeletal chest pain, musculoskeletal stiffness, non-cardiac chest pain, pain in jaw
- è Includes decreased appetite, early satiety
- ð Includes proteinuria, protein urine present, hemoglobinuria
- ø Includes urinary tract infection, cystitis, pyelonephritis
- ý Includes palmar-plantar erythrodysesthesia syndrome, palmar erythema, plantar erythema
- £ Includes rash, rash maculo-papular, rash pruritic, rash erythematous, rash macular, rash pustular, rash papular, rash vesicular, application site rash
Endocrine Hypothyroidism† 67 0.9 0.9 0 Vascular Hypertension‡ 67 39 6 2.5 Hemorrhagic events§ 25 2.6 15 0.9 General Fatigue¶ 58 11 54 6 Gastrointestinal Diarrhea# 55 8 20 2.8 Nausea 49 2.9 47 1.5 Vomiting 37 2.3 21 2.2 StomatitisÞ 35 2.6 26 1.2 Abdominal painß 34 2.6 21 1.2 Constipation 27 0 25 0.6 Musculoskeletal and Connective Tissue Musculoskeletal disordersà 53 5 27 0.6 Metabolism Decreased appetiteè 44 7 21 0 Investigations Weight loss 34 10 6 0.3 Renal and Urinary Proteinuriað 29 6 3.4 0.3 Infections Urinary tract infectionø 31 5 13 1.2 Nervous System Headache 26 0.6 9 0.3 Respiratory, Thoracic and Mediastinal Dysphonia 22 0 0.6 0 Skin and Subcutaneous Tissue Palmar-plantar erythrodysesthesiaý 23 2.9 0.9 0 Rash£ 20 2.3 4.9 0 Table 52: Laboratory Abnormalities Worsened from Baseline* Occurring in ≥20% (All Grades) or ≥3% (Grades 3-4) of Patients with Endometrial Carcinoma in KEYNOTE-775 Endometrial Carcinoma (pMMR or not MSI-H) Laboratory Test† Intravenous Pembrolizumab
200 mg every 3 weeks
and LenvatinibDoxorubicin or
PaclitaxelAll Grades‡
%Grades 3-4
%All Grades‡
%Grades 3-4
%- * With at least one grade increase from baseline
- † Laboratory abnormality percentage is based on the number of patients who had both baseline and at least one post-baseline laboratory measurement for each parameter: intravenous pembrolizumab and lenvatinib (range: 263 to 340 patients) and doxorubicin or paclitaxel (range: 240 to 322 patients).
- ‡ Graded per NCI CTCAE v4.03
Chemistry Hypertriglyceridemia 70 6 45 1.7 Hypoalbuminemia 60 2.7 42 1.6 Increased aspartate aminotransferase 58 9 23 1.6 Hyperglycemia 58 8 45 4.4 Hypomagnesemia 46 0 27 1.3 Increased alanine aminotransferase 55 9 21 1.2 Hypercholesterolemia 53 3.2 23 0.7 Hyponatremia 46 15 28 7 Increased alkaline phosphatase 43 4.7 18 0.9 Hypocalcemia 40 4.7 21 1.9 Increased lipase 36 14 13 3.9 Increased creatinine 35 4.7 18 1.9 Hypokalemia 34 10 24 5 Hypophosphatemia 26 8 17 3.2 Increased amylase 25 7 8 1 Hyperkalemia 23 2.4 12 1.2 Increased creatine kinase 19 3.7 7 0 Increased bilirubin 18 3.6 6 1.6 Hematology Lymphopenia 51 18 66 23 Thrombocytopenia 50 8 30 4.7 Anemia 49 8 84 14 Leukopenia 43 3.5 83 43 Neutropenia 34 8 80 60 As a Single Agent for the Treatment of Advanced MSI-H or dMMR Endometrial Carcinoma
Among the 90 patients with MSI-H or dMMR endometrial carcinoma enrolled in KEYNOTE-158 [see Clinical Studies (14.16)] treated with intravenous pembrolizumab as a single agent, the median duration of exposure to intravenous pembrolizumab was 8.3 months (range: 1 day to 26.9 months). Adverse reactions occurring in patients with endometrial carcinoma were similar to those occurring in 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent.
TMB-H Cancer
The safety of intravenous pembrolizumab was investigated in 105 patients with TMB-H cancer enrolled in KEYNOTE-158 [see Clinical Studies (14.17)]. The median duration of exposure to intravenous pembrolizumab was 4.9 months (range: 0.03 to 35.2 months). Adverse reactions occurring in patients with TMB-H cancer were similar to those occurring in patients with other solid tumors who received intravenous pembrolizumab as a single agent.
cSCC
Among the 159 patients with advanced cSCC (recurrent or metastatic or locally advanced disease) enrolled in KEYNOTE-629 [see Clinical Studies (14.18)], the median duration of exposure to intravenous pembrolizumab was 6.9 months (range 1 day to 28.9 months). Patients with autoimmune disease or a medical condition that required systemic corticosteroids or other immunosuppressive medications were ineligible. Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC were similar to those occurring in 2799 patients with melanoma or NSCLC treated with intravenous pembrolizumab as a single agent. Laboratory abnormalities (Grades 3-4) that occurred at a higher incidence included lymphopenia (10%) and decreased sodium (10%).
TNBC
Neoadjuvant and Adjuvant Treatment of High-Risk Early-Stage TNBC
The safety of intravenous pembrolizumab in combination with neoadjuvant chemotherapy (carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide) followed by surgery and continued adjuvant treatment with intravenous pembrolizumab as a single agent was investigated in KEYNOTE-522, a randomized (2:1), multicenter, double-blind, placebo-controlled trial in patients with newly diagnosed, previously untreated, high-risk early-stage TNBC.
A total of 778 patients on the intravenous pembrolizumab arm received at least 1 dose of intravenous pembrolizumab in combination with neoadjuvant chemotherapy followed by intravenous pembrolizumab as adjuvant treatment after surgery, compared to 389 patients who received at least 1 dose of placebo in combination with neoadjuvant chemotherapy followed by placebo as adjuvant treatment after surgery [see Clinical Studies (14.19)].
The median duration of exposure to intravenous pembrolizumab 200 mg every 3 weeks was 13.3 months (range: 1 day to 21.9 months).
Fatal adverse reactions occurred in 0.9% of patients receiving intravenous pembrolizumab, including 1 each of adrenal crisis, autoimmune encephalitis, hepatitis, pneumonia, pneumonitis, pulmonary embolism, and sepsis in association with multiple organ dysfunction syndrome and myocardial infarction.
Serious adverse reactions occurred in 44% of patients receiving intravenous pembrolizumab. Serious adverse reactions in ≥2% of patients who received intravenous pembrolizumab included febrile neutropenia (15%), pyrexia (3.7%), anemia (2.6%), and neutropenia (2.2%).
Intravenous pembrolizumab was discontinued for adverse reactions in 20% of patients. The most common adverse reactions (≥1%) resulting in permanent discontinuation of intravenous pembrolizumab were increased ALT (2.7%), increased AST (1.5%), and rash (1%). Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 57% of patients. The most common adverse reactions leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (26%), thrombocytopenia (6%), increased ALT (6%), increased AST (3.7%), anemia (3.5%), rash (3.2%), febrile neutropenia (2.8%), leukopenia (2.8%), upper respiratory tract infection (2.6%), pyrexia (2.2%), and fatigue (2.1%).
Tables 53 and 54 summarize the adverse reactions and laboratory abnormalities, respectively, in patients treated with intravenous pembrolizumab in KEYNOTE-522.
Table 53: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-522 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
with chemotherapy*/ Intravenous Pembrolizumab
n=778Placebo
with chemotherapy*/Placebo
n=389All Grades†
(%)Grades 3-4
(%)All Grades†
(%)Grades 3-4
(%)- * Chemotherapy: carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide
- † Graded per NCI CTCAE v4.0
- ‡ Includes asthenia, fatigue
- § Includes aphthous ulcer, cheilitis, lip pain, lip ulceration, mouth ulceration, mucosal inflammation, oral mucosal eruption, oral pain, stomatitis, tongue blistering, tongue ulceration
- ¶ Includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness
- # Includes dermatitis, dermatitis acneiform, dermatitis allergic, dermatitis bullous, dermatitis exfoliative generalized, drug eruption, eczema, incision site rash, injection site rash, rash, rash erythematous, rash follicular, rash macular, rash maculo-papular, rash morbilliform, rash papular, rash pruritic, rash pustular, rash rubelliform, skin exfoliation, skin toxicity, toxic skin eruption, urticaria, vasculitic rash, viral rash
- Þ Includes neuropathy peripheral, peripheral motor neuropathy, peripheral sensorimotor neuropathy, peripheral sensory neuropathy
- ß Includes cough, productive cough, upper-airway cough syndrome
General Fatigue‡ 70 8 66 3.9 Pyrexia 28 1.3 19 0.3 Gastrointestinal Nausea 67 3.7 66 1.8 Constipation 42 0 39 0.3 Diarrhea 41 3.2 34 1.8 Stomatitis§ 34 2.7 29 1 Vomiting 31 2.7 28 1.5 Abdominal pain¶ 24 0.5 23 0.8 Skin and Subcutaneous Tissue Alopecia 61 0 58 0 Rash# 52 5 41 0.5 Nervous System Peripheral neuropathyÞ 41 3.3 42 2.3 Headache 30 0.5 29 1 Musculoskeletal and Connective Tissue Arthralgia 29 0.5 31 0.3 Myalgia 20 0.5 19 0 Respiratory, Thoracic and Mediastinal Coughß 26 0.1 24 0 Metabolism and Nutrition Decreased appetite 23 0.9 17 0.3 Psychiatric Insomnia 21 0.5 19 0 Table 54: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab in KEYNOTE-522 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
with chemotherapy†/Intravenous PembrolizumabPlacebo
with chemotherapy†/PlaceboAll Grades‡
%Grades 3-4
%All Grades‡
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab in combination with chemotherapy followed by intravenous pembrolizumab as a single agent (range: 762 to 777 patients) and placebo in combination with chemotherapy followed by placebo (range: 381 to 389 patients).
- † Chemotherapy: carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide
- ‡ Graded per NCI CTCAE v4.0
Hematology Anemia 97 22 96 19 Leukopenia 93 41 91 32 Neutropenia 88 62 89 62 Lymphopenia 79 28 74 22 Thrombocytopenia 57 10 56 8 Chemistry Increased ALT 70 9 67 3.9 Increased AST 65 6 56 1.5 Hyperglycemia 63 4.3 61 2.8 Increased alkaline phosphatase 37 1 35 0.5 Hyponatremia 35 9 25 4.6 Hypoalbuminemia 34 1.0 30 1.3 Hypocalcemia 31 2.2 28 3.1 Hypokalemia 31 6 22 2.8 Hypophosphatemia 20 6 15 4.2 Locally Recurrent Unresectable or Metastatic TNBC
The safety of intravenous pembrolizumab in combination with paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin was investigated in KEYNOTE-355, a multicenter, double-blind, randomized (2:1), placebo-controlled trial in patients with locally recurrent unresectable or metastatic TNBC who had not been previously treated with chemotherapy in the metastatic setting [see Clinical Studies (14.19)]. A total of 596 patients (including 34 patients from a safety run-in) received intravenous pembrolizumab 200 mg every 3 weeks in combination with paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin.
The median duration of exposure to intravenous pembrolizumab was 5.7 months (range: 1 day to 33.0 months).
Fatal adverse reactions occurred in 2.5% of patients receiving intravenous pembrolizumab in combination with chemotherapy, including cardio-respiratory arrest (0.7%) and septic shock (0.3%).
Serious adverse reactions occurred in 30% of patients receiving intravenous pembrolizumab in combination with paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin. Serious adverse reactions in ≥2% of patients were pneumonia (2.9%), anemia (2.2%), and thrombocytopenia (2%).
Intravenous pembrolizumab was discontinued for adverse reactions in 11% of patients. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab (≥1%) were increased ALT (2.2%), increased AST (1.5%), and pneumonitis (1.2%). Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 50% of patients. The most common adverse reactions leading to interruption of intravenous pembrolizumab (≥2%) were neutropenia (22%), thrombocytopenia (14%), anemia (7%), increased ALT (6%), leukopenia (5%), increased AST (5%), decreased white blood cell count (3.9%), and diarrhea (2%).
Tables 55 and 56 summarize the adverse reactions and laboratory abnormalities in patients on intravenous pembrolizumab in KEYNOTE-355.
Table 55: Adverse Reactions Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab with Chemotherapy in KEYNOTE-355 Adverse Reaction Intravenous Pembrolizumab
200 mg every 3 weeks
with chemotherapy
n=596Placebo
every 3 weeks
with chemotherapy
n=281All Grades*
(%)Grades 3-4
(%)All Grades*
(%)Grades 3-4
(%)- * Graded per NCI CTCAE v4.03
- † Includes fatigue and asthenia
- ‡ Includes rash, rash maculo-papular, rash pruritic, rash pustular, rash macular, rash papular, butterfly rash, rash erythematous, eyelid rash
- § Includes cough, productive cough, upper-airway cough syndrome
- ¶ Includes headache, migraine, tension headache
General Fatigue† 48 5 49 4.3 Gastrointestinal Nausea 44 1.7 47 1.8 Diarrhea 28 1.8 23 1.8 Constipation 28 0.5 27 0.4 Vomiting 26 2.7 22 3.2 Skin and Subcutaneous Tissue Alopecia 34 0.8 35 1.1 Rash‡ 26 2 16 0 Respiratory, Thoracic and Mediastinal Cough§ 23 0 20 0.4 Metabolism and Nutrition Decreased appetite 21 0.8 14 0.4 Nervous System Headache¶ 20 0.7 23 0.7 Table 56: Laboratory Abnormalities Worsened from Baseline Occurring in ≥20% of Patients Receiving Intravenous Pembrolizumab with Chemotherapy in KEYNOTE-355 Laboratory Test* Intravenous Pembrolizumab
200 mg every 3 weeks
with chemotherapyPlacebo
every 3 weeks
with chemotherapyAll Grades†
%Grades 3-4
%All Grades†
%Grades 3-4
%- * Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: intravenous pembrolizumab + chemotherapy (range: 566 to 592 patients) and placebo + chemotherapy (range: 269 to 280 patients).
- † Graded per NCI CTCAE v4.03
Hematology Anemia 90 20 85 19 Leukopenia 85 39 86 39 Neutropenia 78 50 79 53 Lymphopenia 73 28 71 19 Thrombocytopenia 54 19 53 21 Chemistry Increased ALT 60 11 58 8 Increased AST 57 9 55 6 Hyperglycemia 52 4.4 51 2.2 Hypoalbuminemia 36 2.0 32 2.2 Increased alkaline phosphatase 35 3.9 39 2.2 Hypocalcemia 29 3.3 27 1.8 Hyponatremia 28 5 26 6 Hypophosphatemia 21 7 18 4.8 Hypokalemia 20 4.4 18 4.0 Ovarian Cancer
The safety of intravenous pembrolizumab in combination with paclitaxel with or without bevacizumab was evaluated in 463 patients with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma whose tumors express PD-L1 (CPS ≥1) enrolled in KEYNOTE-B96 [see Clinical Studies (14.20)]. Among patients who received intravenous pembrolizumab, the median duration of exposure was 7.4 months (range: 1 day to 35.9 months).
Serious adverse reactions occurred in 54% of patients receiving intravenous pembrolizumab and paclitaxel with or without bevacizumab. Serious adverse reactions in ≥2% of patients were pneumonia (4.3%), urinary tract infection (3.9%), adrenal insufficiency (3%), hyponatremia (3%), COVID-19 (2.6%), decreased neutrophil count (2.6%), pulmonary embolism (2.6%), abdominal pain (2.1%), anemia (2.1%), colitis (2.1%), diarrhea (2.1%), febrile neutropenia (2.1%), pyrexia (2.1%) and vomiting (2.1%).
Fatal adverse reactions occurred in 3.9% of patients receiving intravenous pembrolizumab and paclitaxel with or without bevacizumab, including assisted suicide (0.9%), death (0.4%), intestinal perforation (0.4%), sepsis (0.4%), COVID-19 (0.4%), cardio-respiratory arrest (0.4%), colitis (0.4%), and embolic stroke (0.4%).
Intravenous pembrolizumab was permanently discontinued for adverse reactions in 16% of patients. The most common adverse reactions resulting in permanent discontinuation of intravenous pembrolizumab (≥1%) were colitis (1.3%) and increased alanine aminotransferase (1.3%). Adverse reactions leading to the interruption of intravenous pembrolizumab occurred in 44% of patients. The most common adverse reactions leading to interruption of intravenous pembrolizumab in ≥2% were urinary tract infection (3.9%), adrenal insufficiency (2.6%), pyrexia (2.6%), pneumonitis (2.6%), upper respiratory tract infection (2.6%), neutropenia (2.1%), diarrhea (2.1%) and COVID-19 (2.1%).
The most common (≥20%) adverse reactions for patients treated with intravenous pembrolizumab in combination with paclitaxel with or without bevacizumab were: diarrhea (45%), fatigue (43%), nausea (41%), alopecia (38%), peripheral neuropathy (38%), epistaxis (31%), urinary tract infection (27%), constipation (25%), abdominal pain (24%), decreased appetite (24%), vomiting (24%), hypothyroidism (21%), cough (20%), hypertension (20%), and rash (20%). The most common (≥20%) laboratory abnormalities worsening from baseline were: anemia (85%), leukopenia (82%), decreased neutrophil count (71%), lymphopenia (60%), hypoalbuminemia (50%), hyponatremia (53%), hypomagnesemia (45%), increased aspartate aminotransferase (43%), increased alanine aminotransferase (40%), hypocalcemia(40%), increased alkaline phosphatase (31%), increased creatinine (29%), hypokalemia (27%) and neutropenia (21%).
For patients treated with intravenous pembrolizumab in combination with paclitaxel and bevacizumab (N=169), decreased white blood cell count (27%), stomatitis (22%) and pyrexia (21%) were also reported as adverse reactions.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of intravenous pembrolizumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal: Exocrine pancreatic insufficiency
Hepatobiliary: sclerosing cholangitis
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [see Clinical Pharmacology (12.1)], KEYTRUDA QLEX can cause fetal harm when administered to a pregnant woman. There are no available human data informing the risk of embryo-fetal toxicity. In animal models, the PD-1/PD-L1 signaling pathway is important in the maintenance of pregnancy through induction of maternal immune tolerance to fetal tissue (see Data). Human IgG4 (immunoglobulins) are known to cross the placenta; therefore, pembrolizumab has the potential to be transmitted from the mother to the developing fetus. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
KEYTRUDA QLEX for subcutaneous injection contains pembrolizumab and berahyaluronidase alfa [see Description (11)].
Pembrolizumab:
Animal reproduction studies have not been conducted with pembrolizumab to evaluate its effect on reproduction and fetal development. A literature-based assessment of the effects of the PD-1 pathway on reproduction demonstrated that a central function of the PD-1/PD-L1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to the fetus. Blockade of PD-L1 signaling has been shown in murine models of pregnancy to disrupt tolerance to the fetus and to result in an increase in fetal loss; therefore, potential risks of administering pembrolizumab during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 knockout mice. Based on its mechanism of action, fetal exposure to pembrolizumab may increase the risk of developing immune-mediated disorders or of altering the normal immune response.
Berahyaluronidase alfa:
In an embryo-fetal development study, pregnant rabbits were administered daily subcutaneous injections of 138,600, 403,200, or 1,209,600 U/kg berahyaluronidase alfa during the period of organogenesis (gestation days 6 to 19). Berahyaluronidase alfa caused delayed fetal development at doses ≥403,200 U/kg, which is >2,500 times higher than the human dose (U/kg basis). Increased post-implantation loss and visceral malformations (supernumerary fissure lung lobe) were observed at 1,209,600 U/kg, which is >7,500 times higher than the human dose. In an embryo-fetal development study in rats, there were no adverse embryo-fetal findings in pregnant animals administered daily subcutaneous injections of berahyaluronidase alfa at doses up to 2,520,000 U/kg (>15,000 times higher than the human dose) during the period of organogenesis (gestation days 6 to 17).
In a pre- and post-natal development study in rats, pregnant animals were administered daily subcutaneous injections of 280,000, 840,000, or 2,520,000 U/kg berahyaluronidase alfa from implantation through lactation and weaning (gestation day 6 to lactation day 21). There were no adverse effects on sexual maturation, learning and memory, or fertility of the offspring at doses up to 2,520,000 U/kg, which is >15,000 times higher than the human dose.
8.2 Lactation
Risk Summary
There are no data on the presence of pembrolizumab or berahyaluronidase alfa in either animal or human milk or its effects on the breastfed child or on milk production. Maternal IgG is known to be present in human milk.
The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to KEYTRUDA QLEX are unknown. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with KEYTRUDA QLEX and for 4 months after the last dose.
8.3 Females and Males of Reproductive Potential
Based on its mechanism of action, KEYTRUDA QLEX can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating KEYTRUDA QLEX [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of KEYTRUDA QLEX for the treatment of pediatric patients 12 years and older who weigh greater than 40 kg have been established for:
- Stage IIB, IIC, or III melanoma following complete resection [see Indications and Usage (1.1)]
- Unresectable or metastatic microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) solid tumors [see Indications and Usage (1.6)]
- Recurrent locally advanced or metastatic Merkel cell carcinoma [see Indications and Usage (1.13)]
- Unresectable or metastatic tumor mutational burden high solid tumors (TMB-H) [see Indications and Usage (1.16)]
Use of KEYTRUDA QLEX in pediatric patients for these indications is supported by evidence from adequate and well-controlled studies of intravenous pembrolizumab in adults and additional pharmacokinetic and safety data for intravenous pembrolizumab in pediatric patients 12 years and older [see Adverse Reactions (6.1), Clinical Studies (14)]. Pembrolizumab exposures in pediatric patients 12 years and older who weigh greater than 40 kg are predicted to be within range of those observed in adults at the same dosage [see Clinical Pharmacology (12.3)].
The safety and effectiveness of KEYTRUDA QLEX have not been established in pediatric patients younger than 12 years of age for the treatment of melanoma, MCC, MSI-H or dMMR cancer, and TMB-H cancer.
The safety and effectiveness of KEYTRUDA QLEX have not been established in pediatric patients for other approved indications [see Indications and Usage (1)].
Intravenous pembrolizumab
In KEYNOTE-051, 173 pediatric patients (including 108 pediatric patients aged 12 to 17 years) with advanced melanoma, lymphoma, or PD-L1 positive or MSI-H solid tumors received intravenous pembrolizumab 2 mg/kg every 3 weeks. The median duration of exposure was 2.1 months (range: 1 day to 25 months). Adverse reactions that occurred at a ≥10% higher rate in pediatric patients when compared to adults included pyrexia (33%), vomiting (29%), headache (25%), abdominal pain (23%), decreased lymphocyte count (13%), and decreased white blood cell count (11%). Laboratory abnormalities that occurred at a ≥10% higher rate in pediatric patients when compared to adults were leukopenia (30%), neutropenia (28%), thrombocytopenia (22%), and Grade 3 anemia (17%).
8.5 Geriatric Use
Of the 251 patients treated with KEYTRUDA QLEX in combination with platinum doublet chemotherapy in Study MK-3745A-D77, 53% were 65 years and older and 16% were 75 years and older. No overall differences in safety or effectiveness of KEYTRUDA QLEX have been observed between patients aged 65 years or older and younger adult patients.
The safety of KEYTRUDA QLEX as monotherapy or in combination with other antineoplastic drugs for its approved indications [see Indications and Usage (1)] has been established in adequate and well-controlled studies of intravenous pembrolizumab as a single agent and in combination with other antineoplastic drugs. Below is a description of geriatric use information from the intravenous pembrolizumab studies.
- Of 3781 patients with melanoma, NSCLC, HNSCC, or urothelial carcinoma who were treated with intravenous pembrolizumab in clinical studies, 48% were 65 years and over and 17% were 75 years and over.
- Of 506 adult patients with Stage IB (T2a ≥4 cm), II, or IIIA NSCLC following complete resection and platinum-based chemotherapy who were treated with intravenous pembrolizumab in KEYNOTE-091, 242 (48%) were 65 years and over.
- Of 596 adult patients with TNBC who were treated with intravenous pembrolizumab in combination with paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin in KEYNOTE-355, 137 (23%) were 65 years and over.
- Of 406 adult patients with endometrial carcinoma who were treated with intravenous pembrolizumab in combination with lenvatinib in KEYNOTE-775, 201 (50%) were 65 years and over.
- Of the 564 patients with locally advanced or metastatic urothelial cancer treated with intravenous pembrolizumab in combination with enfortumab vedotin, 44% (n=247) were 65-74 years and 26% (n=144) were 75 years or older. No overall differences in effectiveness were observed between patients 65 years of age or older and younger patients. Patients 75 years of age or older treated with intravenous pembrolizumab in combination with enfortumab vedotin experienced a higher incidence of fatal adverse reactions than younger patients. The incidence of fatal adverse reactions was 4% in patients younger than 75 and 7% in patients 75 years or older.
- Of the 167 patients with MIBC treated with intravenous pembrolizumab in combination with enfortumab vedotin, 37% (n=61) were 65-74 years and 46% (n=77) were 75 years or older. Patients 75 years of age or older treated with intravenous pembrolizumab in combination with enfortumab vedotin experienced a higher incidence of fatal adverse reactions than younger patients. The incidence of fatal adverse reactions was 4% in patients younger than 75 and 12% in patients 75 years or older.
- Of the 432 patients randomized to intravenous pembrolizumab in combination with axitinib in the KEYNOTE-426 trial, 40% were 65 years or older.
- Of 294 adult patients with FIGO 2014 Stage III-IVA cervical cancer who were treated with intravenous pembrolizumab in combination with CRT in KEYNOTE-A18, 42 (14%) were 65 years and over.
- Of 643 adult patients with ovarian cancer who were treated with intravenous pembrolizumab in combination with paclitaxel with or without bevacizumab in KEYNOTE-B96, 236 (37%) were 65 years and over and 58 (9%) were 75 years and over.
No overall differences in safety or effectiveness were observed between intravenous pembrolizumab-treated patients aged 65 years or older and younger adult patients.
-
11 DESCRIPTION
KEYTRUDA QLEX is a fixed-combination drug product containing pembrolizumab and berahyaluronidase alfa.
Pembrolizumab is a programmed death receptor-1 (PD 1)-blocking antibody. Pembrolizumab is a humanized monoclonal IgG4 kappa antibody with an approximate molecular weight of 149 kDa. Pembrolizumab is produced in recombinant Chinese hamster ovary (CHO) cells.
Berahyaluronidase alfa is an endoglycosidase used to enhance dispersion and permeation, which facilitates delivery of increased volume of pembrolizumab that is co-administered subcutaneously. It is produced by mammalian CHO (Chinese Hamster Ovary) cells containing a DNA plasmid encoding a variant of human hyaluronidase PH20. It is a glycosylated protein with an approximate molecular weight of 49 kDa under nonreducing, deglycosylated conditions.
KEYTRUDA QLEX (pembrolizumab and berahyaluronidase alfa-pmph) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution supplied in single-dose vials for subcutaneous administration.
KEYTRUDA QLEX is supplied as two different configurations:
- Each KEYTRUDA QLEX 2.4 mL single-dose vial contains 395 mg of pembrolizumab and 4,800 units of berahyaluronidase alfa, and histidine (0.7 mg), histidine hydrochloride monohydrate (4.1 mg), methionine (3.6 mg), polysorbate 80 (0.5 mg), sucrose (168 mg), and Water for Injection, USP. The pH is 5.3-5.9.
- Each KEYTRUDA QLEX 4.8 mL single-dose vial contains 790 mg of pembrolizumab and 9,600 units of berahyaluronidase alfa, and histidine (1.4 mg), histidine hydrochloride monohydrate (8.2 mg), methionine (7.2 mg), polysorbate 80 (1 mg), sucrose (336 mg), and Water for Injection, USP. The pH is 5.3-5.9.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
In syngeneic mouse tumor models, combination treatment of a PD-1 blocking antibody and kinase inhibitor lenvatinib decreased tumor-associated macrophages, increased activated cytotoxic T cells, and reduced tumor growth compared to either treatment alone.
Berahyaluronidase alfa, an endoglycosidase, is a variant of human hyaluronidase PH20 that temporarily and locally breaks down hyaluronan. Hyaluronan is a polysaccharide found in the extracellular matrix of the subcutaneous tissue. Unlike the stable structural components of the interstitial matrix, hyaluronan has a half-life of approximately 0.5 days. Hyaluronidase increases permeability of the subcutaneous tissue by depolymerizing hyaluronan.
In the doses administered, the effects of berahyaluronidase alfa are reversible and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
12.2 Pharmacodynamics
There are no clinically significant exposure-response relationships for efficacy or safety for intravenous pembrolizumab across the approved dosing regimens, regardless of cancer type. The exposures from subcutaneous KEYTRUDA QLEX doses of 395 mg/4,800 units every 3 weeks or 790 mg/9,600 units every 6 weeks are within the range of exposures from intravenous pembrolizumab doses.
12.3 Pharmacokinetics
Pembrolizumab pharmacokinetics were characterized at Cycle 1 and at steady state in patients with advanced solid tumors at the approved recommended dosages and are presented as mean (CV%) unless otherwise specified.
When comparing pembrolizumab exposure following subcutaneous administration every 6 weeks to that of intravenous administration every 6 weeks in Study MK-3475A-D77 [see Clinical Studies (14.1)], the geometric mean ratio (GMR) for Cycle 1 AUC0-6wks was 1.14 (96% CI: 1.06, 1.22) and Cycle 3 Ctrough (i.e., steady state) was 1.67 (94% CI: 1.52, 1.84).
Pembrolizumab steady state was reached by 16 weeks. At steady state following subcutaneous administration, the mean pembrolizumab AUC0-6wks was 2,798 mcgday/mL for the every 6 week dosing and pembrolizumab AUC0-3wks was 1,343 mcgday/mL for the every 3 week dosing. Pembrolizumab Ctrough was 39 mcg/mL for the every 6 week dosing and 49 mcg/mL for the every 3 week dosing.
The systemic accumulation ratio was 1.6-fold following administration of KEYTRUDA QLEX 790 mg/9,600 units every 6 weeks and 2.5-fold following administration of KEYTRUDA QLEX 395 mg/4,800 units every 3 weeks.
Absorption
Pembrolizumab bioavailability (CV%) is approximately 60% (14%). Peak concentrations occurred by approximately 4 days.
Distribution
The volume of distribution is 6 L.
Elimination
Pembrolizumab clearance decreases over time, resulting in a steady state clearance (CV%) of - 195 mL/day (40%); this decrease in clearance with time is not considered clinically significant. The terminal half-life is 22 days.
Specific Populations
No clinically significant differences in the pharmacokinetics of pembrolizumab were observed based on age (37 to 87 years), race (63% White, 28% Asian, 3% Black), sex, body weight (37 to 144 kg), tumor type, injection site (thigh or abdomen), estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2, and mild to moderate hepatic impairment (total bilirubin ≤3 times ULN and any AST). The effect of severe hepatic impairment (total bilirubin >3 times ULN and any AST) on pembrolizumab pharmacokinetics is unknown.
Pediatric Patients
Pembrolizumab exposures in pediatric patients 12 years and older who weigh greater than 40 kg are predicted to be within range of those observed in adult patients at the same dosage.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described in this section with the incidence of ADA in other studies, including those of KEYTRUDA QLEX or of other pembrolizumab products or berahyaluronidase alfa products.
With a median (min, max) duration of treatment on KEYTRUDA QLEX of 6.9 months (1 day, 1 year) in Study MK-3475A-D77, 1.4% (3/211) of patients developed anti-pembrolizumab antibodies, and one ADA-positive patient developed neutralizing antibodies (NAb) against pembrolizumab. The incidence of anti-berahyaluronidase alfa antibodies was 1.5% (3/194). No analysis of neutralizing antibodies was performed for berahyaluronidase alfa ADA-positive samples. Because of the low occurrence of anti-pembrolizumab or anti-berahyaluronidase antibodies, the effect of these antibodies on the pharmacokinetics, safety and effectiveness of KEYTRUDA QLEX is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
KEYTRUDA QLEX contains pembrolizumab and berahyaluronidase alfa.
Pembrolizumab
No studies have been performed to test the potential of pembrolizumab for carcinogenicity or genotoxicity.
Fertility studies have not been conducted with pembrolizumab. In 1-month and 6-month repeat-dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs; however, most animals in these studies were not sexually mature.
Berahyaluronidase alfa
Hyaluronidases are found in most tissues of the body. Long-term animal studies have not been performed to assess the carcinogenic or mutagenic potential of berahyaluronidase alfa.
In a fertility and early embryonic development study, male and female rats were administered daily subcutaneous injections of 280,000, 840,000, or 2,520,000 U/kg berahyaluronidase alfa. Males were dosed for 9 weeks prior to mating and throughout mating to termination. Females were dosed for 2 weeks prior to mating, throughout mating, and up to gestation day 7. Although treatment with ≥840,000 U/kg berahyaluronidase alfa (>5,200 times higher than the human dose) resulted in an increased incidence of abnormal sperm morphology, there were no adverse effects on mating, fertility or embryogenesis observed at doses up to 2,520,000 U/kg (>15,000 times higher than the human dose).
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-1/PD-L1 signaling increased the severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti-PD-1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-1 and PD-L1 knockout mice and mice receiving PD-L1-blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus. Administration of pembrolizumab in chimpanzees with naturally occurring chronic hepatitis B infection resulted in two out of four animals with significantly increased levels of serum ALT, AST, and GGT, which persisted for at least 1 month after discontinuation of pembrolizumab.
-
14 CLINICAL STUDIES
14.1 KEYTRUDA QLEX
NSCLC (Study MK-3475A-D77)
KEYTRUDA QLEX was evaluated in Study MK-3475A-D77 (NCT05722015), a randomized, multicenter, open-label, active-controlled trial conducted in patients with treatment-naïve metastatic NSCLC, in whom there were no EGFR, ALK, or ROS1 genomic tumor aberrations. Patients were excluded if they had a history of autoimmune disease that required systemic therapy within 2 years of treatment; had a medical condition that required immunosuppression; or had received more than 30 Gy of thoracic radiation within the prior 26 weeks.
A total of 377 patients were randomized (2:1) to receive either KEYTRUDA QLEX (containing 790 mg pembrolizumab and 9,600 units berahyaluronidase alfa) administered subcutaneously every 6 weeks with platinum doublet chemotherapy (n=251) or pembrolizumab 400 mg intravenously every 6 weeks with platinum doublet chemotherapy (n=126).
The chemotherapy regimens were as follows:
- Non-squamous NSCLC: pemetrexed 500 mg/m2 and a platinum chemotherapy (cisplatin 75 mg/m2 or carboplatin AUC 5 mg/mL/min) intravenously every 3 weeks for 4 cycles, followed by pemetrexed 500 mg/m2 intravenously every 3 weeks.
- Squamous NSCLC: carboplatin AUC 6 mg/mL/min and a taxane (paclitaxel 200 mg/m2 on Day 1 of each 21-day cycle or paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 of each 21-day cycle) intravenously every 3 weeks for 4 cycles.
Randomization was stratified by ECOG performance status (0 vs. 1), histology (squamous vs. non-squamous), PD-L1 TPS (<50% vs. ≥50%), and geographic region (East Asia vs. North America/Western Europe/Australia/New Zealand vs. Rest of the World).
Treatment with KEYTRUDA QLEX or intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 18 cycles (approximately 24 months). Administration of KEYTRUDA QLEX or intravenous pembrolizumab was permitted beyond RECIST-defined disease progression by BICR or beyond discontinuation of pemetrexed if the patient was clinically stable and deriving clinical benefit as determined by the investigator.
The primary outcome measure was pembrolizumab exposure [Cycle 1 AUC0-6 weeks and Cycle 3 (i.e., Steady State) Ctrough] of subcutaneous KEYTRUDA QLEX as compared to intravenous pembrolizumab [see Clinical Pharmacology (12.3)]. Additional descriptive efficacy outcome measures were overall response rate (ORR) by blinded independent central review (BICR), progression-free survival (PFS) by BICR, and overall survival (OS).
The study population characteristics were: median age 65 years (range: 37 to 87); 71% male; 63% White, 29% Asian, 4% multiracial, 3% Black or African American, 2% American Indian or Alaska Native; 31% of Hispanic or Latino ethnicity; 35% ECOG performance status (PS) of 0 and 65% ECOG PS of 1. Nineteen percent had PD-L1 TPS ≥50%; 34% had tumors with squamous histology and 66% had tumors with non-squamous histology; and 9% had brain metastases at baseline.
At the primary analysis, the confirmed ORR was 45% (95% CI: 39, 52) in the subcutaneous KEYTRUDA QLEX arm and 42% (95% CI: 33, 51) in the intravenous pembrolizumab arm. There were no notable differences in PFS or OS observed in patients who received KEYTRUDA QLEX compared to patients who received intravenous pembrolizumab.
Intravenous Pembrolizumab
The effectiveness of KEYTRUDA QLEX for its approved indications [see Indications and Usage (1)] has been established based upon the evidence from the adequate and well-controlled studies conducted with intravenous pembrolizumab [see Clinical Studies (14.2-14.20)] and additional data that demonstrated comparable pharmacokinetic, efficacy, and safety profiles between KEYTRUDA QLEX and intravenous pembrolizumab in Study MK-3475A-D77 [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].
Below is a description of the efficacy results of these adequate and well-controlled studies of intravenous pembrolizumab in these patient populations.
14.2 Melanoma
Ipilimumab-Naive Melanoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-006 (NCT01866319), a randomized (1:1:1), open-label, multicenter, active-controlled trial in 834 patients. Patients were randomized to receive intravenous pembrolizumab at a dose of 10 mg/kg intravenously every 2 weeks or 10 mg/kg intravenously every 3 weeks until disease progression or unacceptable toxicity or to ipilimumab 3 mg/kg intravenously every 3 weeks for 4 doses unless discontinued earlier for disease progression or unacceptable toxicity. Patients with disease progression could receive additional doses of treatment unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at 4 to 6 weeks with repeat imaging. Randomization was stratified by line of therapy (0 vs. 1), ECOG PS (0 vs. 1), and PD-L1 expression (≥1% of tumor cells [positive] vs. <1% of tumor cells [negative]) according to an investigational use only (IUO) assay. Key eligibility criteria were unresectable or metastatic melanoma; no prior ipilimumab; and no more than one prior systemic treatment for metastatic melanoma. Patients with BRAF V600E mutation-positive melanoma were not required to have received prior BRAF inhibitor therapy. Patients with autoimmune disease; a medical condition that required immunosuppression; previous severe hypersensitivity to other monoclonal antibodies; and HIV, hepatitis B or hepatitis C infection, were ineligible. Assessment of tumor status was performed at 12 weeks, then every 6 weeks through Week 48, followed by every 12 weeks thereafter. The major efficacy outcome measures were overall survival (OS) and progression-free survival (PFS; as assessed by blinded independent central review [BICR] using Response Evaluation Criteria in Solid Tumors [RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ]). Additional efficacy outcome measures were objective response rate (ORR) and duration of response (DoR).
The study population characteristics were: median age of 62 years (range: 18 to 89); 60% male; 98% White; 66% had no prior systemic therapy for metastatic disease; 69% ECOG PS of 0; 80% had PD-L1 positive melanoma, 18% had PD-L1 negative melanoma, and 2% had unknown PD-L1 status using the IUO assay; 65% had M1c stage disease; 68% with normal LDH; 36% with reported BRAF mutation-positive melanoma; and 9% with a history of brain metastases. Among patients with BRAF mutation-positive melanoma, 139 (46%) were previously treated with a BRAF inhibitor.
The study demonstrated statistically significant improvements in OS and PFS for patients randomized to intravenous pembrolizumab as compared to ipilimumab. Among the 91 patients randomized to intravenous pembrolizumab 10 mg/kg every 3 weeks with an objective response, response durations ranged from 1.4+ to 8.1+ months. Among the 94 patients randomized to intravenous pembrolizumab 10 mg/kg every 2 weeks with an objective response, response durations ranged from 1.4+ to 8.2 months. Efficacy results are summarized in Table 57 and Figure 1.
Table 57: Efficacy Results in KEYNOTE-006 Endpoint Intravenous Pembrolizumab
10 mg/kg every 3 weeks
n=277Intravenous Pembrolizumab
10 mg/kg every 2 weeks
n=279Ipilimumab
3 mg/kg every 3 weeks
n=278- * Hazard ratio (intravenous pembrolizumab compared to ipilimumab) based on the stratified Cox proportional hazard model
OS Deaths (%) 92 (33%) 85 (30%) 112 (40%) Hazard ratio* (95% CI) 0.69 (0.52, 0.90) 0.63 (0.47, 0.83) --- p-Value (stratified log-rank) 0.004 <0.001 --- PFS by BICR Events (%) 157 (57%) 157 (56%) 188 (68%) Median in months (95% CI) 4.1 (2.9, 6.9) 5.5 (3.4, 6.9) 2.8 (2.8, 2.9) Hazard ratio* (95% CI) 0.58 (0.47, 0.72) 0.58 (0.46, 0.72) --- p-Value (stratified log-rank) <0.001 <0.001 --- Best objective response by BICR ORR (95% CI) 33% (27, 39) 34% (28, 40) 12% (8, 16) Complete response rate 6% 5% 1% Partial response rate 27% 29% 10% Ipilimumab-Refractory Melanoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-002 (NCT01704287), a multicenter, randomized (1:1:1), active-controlled trial in 540 patients randomized to receive one of two doses of intravenous pembrolizumab in a blinded fashion or investigator's choice chemotherapy. The treatment arms consisted of intravenous pembrolizumab 2 mg/kg or 10 mg/kg intravenously every 3 weeks or investigator's choice of any of the following chemotherapy regimens: dacarbazine 1000 mg/m2 intravenously every 3 weeks (26%), temozolomide 200 mg/m2 orally once daily for 5 days every 28 days (25%), carboplatin AUC 6 mg/mL/min intravenously plus paclitaxel 225 mg/m2 intravenously every 3 weeks for four cycles then carboplatin AUC of 5 mg/mL/min plus paclitaxel 175 mg/m2 every 3 weeks (25%), paclitaxel 175 mg/m2 intravenously every 3 weeks (16%), or carboplatin AUC 5 or 6 mg/mL/min intravenously every 3 weeks (8%). Randomization was stratified by ECOG PS (0 vs. 1), LDH levels (normal vs. elevated [≥110% ULN]) and BRAF V600 mutation status (wild-type [WT] or V600E). The trial included patients with unresectable or metastatic melanoma with progression of disease; refractory to two or more doses of ipilimumab (3 mg/kg or higher) and, if BRAF V600 mutation-positive, a BRAF or MEK inhibitor; and disease progression within 24 weeks following the last dose of ipilimumab. The trial excluded patients with uveal melanoma and active brain metastasis. Patients received intravenous pembrolizumab until unacceptable toxicity; disease progression that was symptomatic, was rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at 4 to 6 weeks with repeat imaging; withdrawal of consent; or physician's decision to stop therapy for the patient. Assessment of tumor status was performed at 12 weeks after randomization, then every 6 weeks through week 48, followed by every 12 weeks thereafter. Patients on chemotherapy who experienced progression of disease were offered intravenous pembrolizumab. The major efficacy outcomes were PFS as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and OS. Additional efficacy outcome measures were confirmed ORR as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and DoR.
The study population characteristics were: median age of 62 years (range: 15 to 89), 43% age 65 or older; 61% male; 98% White; and 55% ECOG PS of 0 and 45% ECOG PS of 1. Twenty-three percent of patients were BRAF V600 mutation positive, 40% had elevated LDH at baseline, 82% had M1c disease, and 73% had two or more prior therapies for advanced or metastatic disease.
The study demonstrated a statistically significant improvement in PFS for patients randomized to intravenous pembrolizumab as compared to control arm. There was no statistically significant difference between intravenous pembrolizumab 2 mg/kg and chemotherapy or between intravenous pembrolizumab 10 mg/kg and chemotherapy in the OS analysis in which 55% of the patients who had been randomized to receive chemotherapy had crossed over to receive intravenous pembrolizumab. Among the 38 patients randomized to intravenous pembrolizumab 2 mg/kg with an objective response, response durations ranged from 1.3+ to 11.5+ months. Among the 46 patients randomized to intravenous pembrolizumab 10 mg/kg with an objective response, response durations ranged from 1.1+ to 11.1+ months. Efficacy results are summarized in Table 58 and Figure 2.
Table 58: Efficacy Results in KEYNOTE-002 Endpoint Intravenous Pembrolizumab
2 mg/kg every 3 weeksIntravenous Pembrolizumab
10 mg/kg every 3 weeksChemotherapy n=180 n=181 n=179 - * Hazard ratio (intravenous pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
- † With additional follow-up of 18 months after the PFS analysis
- ‡ Not statistically significant compared to multiplicity adjusted significance level of 0.01
PFS Number of Events, n (%) 129 (72%) 126 (70%) 155 (87%) Progression, n (%) 105 (58%) 107 (59%) 134 (75%) Death, n (%) 24 (13%) 19 (10%) 21 (12%) Median in months (95% CI) 2.9 (2.8, 3.8) 2.9 (2.8, 4.7) 2.7 (2.5, 2.8) p-Value (stratified log-rank) <0.001 <0.001 --- Hazard ratio* (95% CI) 0.57 (0.45, 0.73) 0.50 (0.39, 0.64) --- OS† Deaths (%) 123 (68%) 117 (65%) 128 (72%) Hazard ratio* (95% CI) 0.86 (0.67, 1.10) 0.74 (0.57, 0.96) --- p-Value (stratified log-rank) 0.117 0.011‡ --- Median in months (95% CI) 13.4 (11.0, 16.4) 14.7 (11.3, 19.5) 11.0 (8.9, 13.8) Objective Response Rate ORR (95% CI) 21% (15, 28) 25% (19, 32) 4% (2, 9) Complete response rate 2% 3% 0% Partial response rate 19% 23% 4% Figure 2: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-002 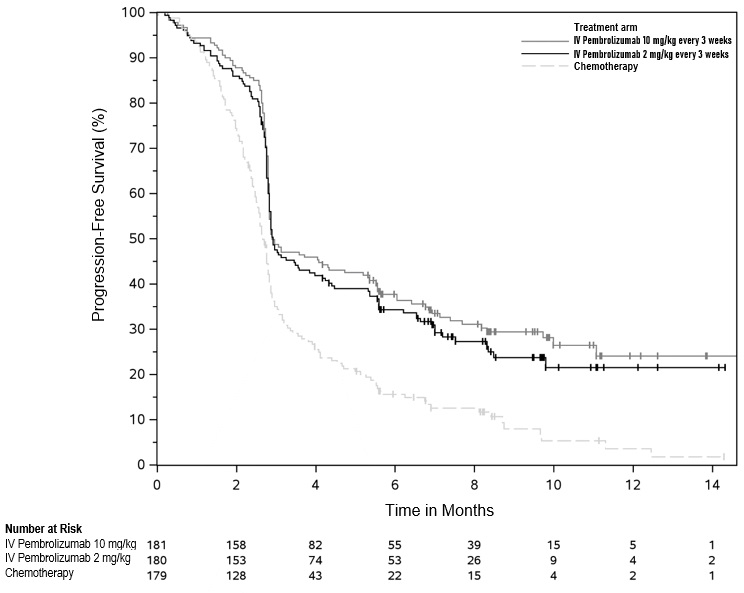
Adjuvant Treatment of Resected Stage IIB or IIC Melanoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-716 (NCT03553836), a multicenter, randomized (1:1), double-blind, placebo-controlled trial in patients with completely resected Stage IIB or IIC melanoma. Patients were randomized to intravenous pembrolizumab 200 mg or the pediatric (≥12 years old) dose of intravenous pembrolizumab 2 mg/kg intravenously (up to a maximum of 200 mg) every three weeks or placebo for up to one year until disease recurrence or unacceptable toxicity. Randomization was stratified by AJCC 8th edition T Stage (>2.0-4.0 mm with ulceration vs. >4.0 mm without ulceration vs. >4.0 mm with ulceration). Patients must not have been previously treated for melanoma beyond complete surgical resection for their melanoma prior to study entry. The major efficacy outcome measure was investigator-assessed recurrence-free survival (RFS) (defined as the time between the date of randomization and the date of first recurrence [local, in-transit or regional lymph nodes or distant recurrence] or death, whichever occurred first). New primary melanomas were excluded from the definition of RFS. Distant metastasis-free survival (DMFS), defined as a spread of tumor to distant organs or distant lymph nodes, was an additional efficacy outcome measure. Patients underwent imaging every six months for one year from randomization, every 6 months from years 2 to 4, and then once in year 5 from randomization or until recurrence, whichever came first.
The study population characteristics were: median age of 61 years (range: 16 to 87), 39% age 65 or older; 60% male; 98% White; and 93% ECOG PS of 0 and 7% ECOG PS of 1. Sixty-four percent had Stage IIB and 35% had Stage IIC.
The trial demonstrated a statistically significant improvement in RFS and DMFS for patients randomized to the intravenous pembrolizumab arm compared with placebo. Efficacy results are summarized in Table 59 and Figure 3.
Table 59: Efficacy Results in KEYNOTE-716 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=487Placebo
n=489NR = not reached - * Based on the stratified Cox proportional hazard model
- † Based on a log-rank test stratified by American Joint Committee on Cancer 8th edition (AJCC) stage
- ‡ p-Value is compared with 0.0202 of the allocated alpha for this interim analysis.
- § p-Value is compared with 0.0256 of the allocated alpha for this interim analysis.
RFS Number (%) of patients with event 54 (11%) 82 (17%) Median in months (95% CI) NR (22.6, NR) NR (NR, NR) Hazard ratio*,† (95% CI) 0.65 (0.46, 0.92) p-Value† 0.0132‡ DMFS Number (%) of patients with event 63 (13%) 95 (19%) Median in months (95% CI) NR (NR, NR) NR (NR, NR) Hazard ratio*,† (95% CI) 0.64 (0.47, 0.88) p-Value† 0.0058§ Figure 3: Kaplan-Meier Curve for Recurrence-Free Survival in KEYNOTE-716 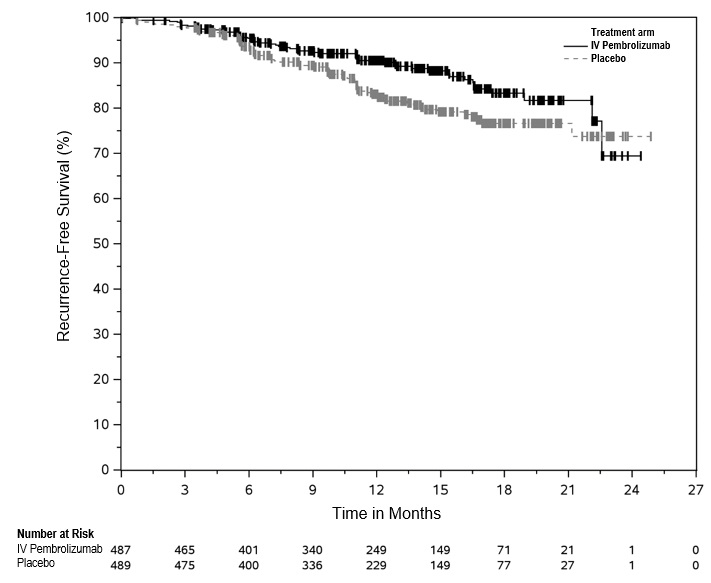
Adjuvant Treatment of Stage III Resected Melanoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-054 (NCT02362594), a multicenter, randomized (1:1), double-blind, placebo-controlled trial in patients with completely resected Stage IIIA (>1 mm lymph node metastasis), IIIB, or IIIC melanoma. Patients were randomized to intravenous pembrolizumab 200 mg intravenously every three weeks or placebo for up to one year until disease recurrence or unacceptable toxicity. Randomization was stratified by American Joint Committee on Cancer 7th edition (AJCC) stage (IIIA vs. IIIB vs. IIIC 1-3 positive lymph nodes vs. IIIC ≥4 positive lymph nodes) and geographic region (North America, European countries, Australia, and other countries as designated). Patients must have undergone lymph node dissection and, if indicated, radiotherapy within 13 weeks prior to starting treatment. The major efficacy outcome measure was investigator-assessed recurrence-free survival (RFS) in the whole population and in the population with PD-L1 positive tumors where RFS was defined as the time between the date of randomization and the date of first recurrence (local, regional, or distant metastasis) or death, whichever occurs first. New primary melanomas were excluded from the definition of RFS. DMFS in the whole population and in the population with PD-L1 positive tumors were additional efficacy outcome measures. DMFS was defined as a spread of tumor to distant organs or distant lymph nodes. Patients underwent imaging every 12 weeks after the first dose of intravenous pembrolizumab for the first two years, then every 6 months from year 3 to 5, and then annually.
The study population characteristics were: median age of 54 years (range: 19 to 88), 25% age 65 or older; 62% male; and 94% ECOG PS of 0 and 6% ECOG PS of 1. Sixteen percent had Stage IIIA, 46% had Stage IIIB, 18% had Stage IIIC (1-3 positive lymph nodes), and 20% had Stage IIIC (≥4 positive lymph nodes); 50% were BRAF V600 mutation positive and 44% were BRAF wild-type; and 84% had PD-L1 positive melanoma with TPS ≥1% according to an IUO assay.
The trial demonstrated a statistically significant improvement in RFS and DMFS for patients randomized to the intravenous pembrolizumab arm compared with placebo. Efficacy results are summarized in Table 60 and Figure 4.
Table 60: Efficacy Results in KEYNOTE-054 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=514Placebo
n=505NR = not reached - * Based on the stratified Cox proportional hazard model
- † Stratified by American Joint Committee on Cancer 7th edition (AJCC) stage
- ‡ p-Value is compared with 0.016 of the allocated alpha for this interim analysis.
- § p-Value is compared with 0.028 of the allocated alpha for this analysis.
RFS Number (%) of patients with event 135 (26%) 216 (43%) Median in months (95% CI) NR 20.4 (16.2, NR) Hazard ratio*,† (95% CI) 0.57 (0.46, 0.70) p-Value† (log-rank) <0.001‡ DMFS Number (%) of patients with event 173 (34%) 245 (49%) Median in months (95% CI) NR (49.6, NR) 40.0 (27.7, NR) Hazard ratio*,† (95% CI) 0.60 (0.49, 0.73) p-Value† (log-rank) <0.0001§ For patients with PD-L1 positive tumors, the RFS HR was 0.54 (95% CI: 0.42, 0.69); p<0.0001. For patients with PD-L1 positive tumors, the DMFS HR was 0.61 (95% CI: 0.49, 0.76); p<0.0001. The RFS and DMFS benefit for intravenous pembrolizumab compared to placebo was observed regardless of tumor PD-L1 expression.
Figure 4: Kaplan-Meier Curve for Recurrence-Free Survival in KEYNOTE-054 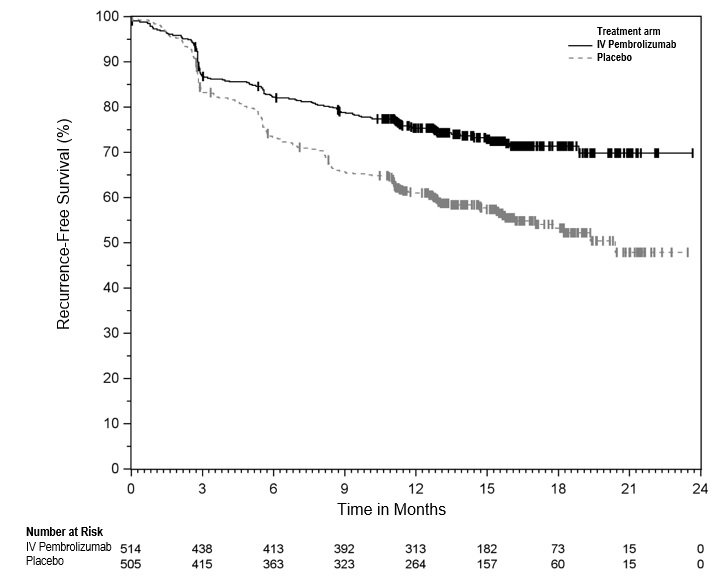
14.3 Non-Small Cell Lung Cancer
First-line treatment of metastatic nonsquamous NSCLC with pemetrexed and platinum chemotherapy
The efficacy of intravenous pembrolizumab in combination with pemetrexed and platinum chemotherapy was investigated in KEYNOTE-189 (NCT02578680), a randomized, multicenter, double-blind, active-controlled trial conducted in 616 patients with metastatic nonsquamous NSCLC, regardless of PD-L1 tumor expression status, who had not previously received systemic therapy for metastatic disease and in whom there were no EGFR or ALK genomic tumor aberrations. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomization was stratified by smoking status (never vs. former/current), choice of platinum (cisplatin vs. carboplatin), and tumor PD-L1 status (TPS <1% [negative] vs. TPS ≥1%). Patients were randomized (2:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg, pemetrexed 500 mg/m2, and investigator's choice of cisplatin 75 mg/m2 or carboplatin AUC 5 mg/mL/min intravenously on Day 1 of each 21-day cycle for 4 cycles followed by intravenous pembrolizumab 200 mg and pemetrexed 500 mg/m2 intravenously every 3 weeks.
- Placebo, pemetrexed 500 mg/m2, and investigator's choice of cisplatin 75 mg/m2 or carboplatin AUC 5 mg/mL/min intravenously on Day 1 of each 21-day cycle for 4 cycles followed by placebo and pemetrexed 500 mg/m2 intravenously every 3 weeks.
Treatment with intravenous pembrolizumab continued until RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Administration of intravenous pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Patients randomized to placebo and chemotherapy were offered intravenous pembrolizumab as a single agent at the time of disease progression. Assessment of tumor status was performed at Week 6, Week 12, and then every 9 weeks thereafter. The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were ORR and DoR, as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 64 years (range: 34 to 84), 49% age 65 or older; 59% male; 94% White and 3% Asian; 56% ECOG PS of 1; and 18% with history of brain metastases. Thirty-one percent had tumor PD-L1 expression TPS <1% [negative]. Seventy-two percent received carboplatin and 12% were never smokers. A total of 85 patients in the placebo and chemotherapy arm received an anti-PD-1/PD-L1 monoclonal antibody at the time of disease progression.
The trial demonstrated a statistically significant improvement in OS and PFS for patients randomized to intravenous pembrolizumab in combination with pemetrexed and platinum chemotherapy compared with placebo, pemetrexed, and platinum chemotherapy. Table 61 and Figure 5 summarize the efficacy results for KEYNOTE-189.
Table 61: Efficacy Results in KEYNOTE-189 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
Pemetrexed
Platinum Chemotherapy
n=410Placebo
Pemetrexed
Platinum Chemotherapy
n=206NR = not reached - * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test
- ‡ Response: Best objective response as confirmed complete response or partial response
- § Based on Miettinen and Nurminen method stratified by PD-L1 status, platinum chemotherapy, and smoking status
OS Number (%) of patients with event 127 (31%) 108 (52%) Median in months (95% CI) NR
(NR, NR)11.3
(8.7, 15.1)Hazard ratio* (95% CI) 0.49 (0.38, 0.64) p-Value† <0.0001 PFS Number of patients with event (%) 245 (60%) 166 (81%) Median in months (95% CI) 8.8 (7.6, 9.2) 4.9 (4.7, 5.5) Hazard ratio* (95% CI) 0.52 (0.43, 0.64) p-Value† <0.0001 Objective Response Rate ORR‡ (95% CI) 48% (43, 53) 19% (14, 25) Complete response 0.5% 0.5% Partial response 47% 18% p-Value§ <0.0001 Duration of Response Median in months (range) 11.2 (1.1+, 18.0+) 7.8 (2.1+, 16.4+) At the protocol-specified final OS analysis, the median in the intravenous pembrolizumab in combination with pemetrexed and platinum chemotherapy arm was 22.0 months (95% CI: 19.5, 24.5) compared to 10.6 months (95% CI: 8.7, 13.6) in the placebo with pemetrexed and platinum chemotherapy arm, with an HR of 0.56 (95% CI: 0.46, 0.69).
First-line treatment of metastatic squamous NSCLC with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy
The efficacy of intravenous pembrolizumab in combination with carboplatin and investigator's choice of either paclitaxel or paclitaxel protein-bound was investigated in KEYNOTE-407 (NCT02775435), a randomized, multi-center, double-blind, placebo-controlled trial conducted in 559 patients with metastatic squamous NSCLC, regardless of PD-L1 tumor expression status, who had not previously received systemic therapy for metastatic disease. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomization was stratified by tumor PD-L1 status (TPS <1% [negative] vs. TPS ≥1%), choice of paclitaxel or paclitaxel protein-bound, and geographic region (East Asia vs. non-East Asia). Patients were randomized (1:1) to one of the following treatment arms; all study medications were administered via intravenous infusion:
- Intravenous pembrolizumab 200 mg and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for 4 cycles, and paclitaxel 200 mg/m2 on Day 1 of each 21-day cycle for 4 cycles or paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 of each 21-day cycle for 4 cycles, followed by intravenous pembrolizumab 200 mg every 3 weeks.
- Placebo and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for 4 cycles and paclitaxel 200 mg/m2 on Day 1 of each 21-day cycle for 4 cycles or paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 of each 21-day cycle for 4 cycles, followed by placebo every 3 weeks.
Treatment with intravenous pembrolizumab and chemotherapy or placebo and chemotherapy continued until RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. Administration of intravenous pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator. Patients randomized to the placebo and chemotherapy arm were offered intravenous pembrolizumab as a single agent at the time of disease progression. Assessment of tumor status was performed every 6 weeks through Week 18, every 9 weeks through Week 45 and every 12 weeks thereafter. The main efficacy outcome measures were PFS and ORR as assessed by BICR using RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and OS. An additional efficacy outcome measure was DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 65 years (range: 29 to 88), 55% age 65 or older; 81% male; 77% White; 71% ECOG PS of 1; and 8% with a history of brain metastases. Thirty-five percent had tumor PD-L1 expression TPS <1%; 19% were from the East Asian region; and 60% received paclitaxel.
The trial demonstrated a statistically significant improvement in OS, PFS and ORR in patients randomized to intravenous pembrolizumab in combination with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy compared with patients randomized to placebo with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy. Table 62 and Figure 6 summarize the efficacy results for KEYNOTE-407.
Table 62: Efficacy Results in KEYNOTE-407 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
Carboplatin
Paclitaxel/Paclitaxel protein-bound
n=278Placebo
Carboplatin
Paclitaxel/Paclitaxel protein-bound
n=281NE = not estimable - * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test
- ‡ ORR primary analysis and DoR analysis were conducted with the first 204 patients enrolled.
- § Based on a stratified Miettinen-Nurminen test
OS Number of events (%) 85 (31%) 120 (43%) Median in months (95% CI) 15.9 (13.2, NE) 11.3 (9.5, 14.8) Hazard ratio* (95% CI) 0.64 (0.49, 0.85) p-Value† 0.0017 PFS Number of events (%) 152 (55%) 197 (70%) Median in months (95% CI) 6.4 (6.2, 8.3) 4.8 (4.2, 5.7) Hazard ratio* (95% CI) 0.56 (0.45, 0.70) p-Value† <0.0001 n=101 n=103 Objective Response Rate‡ ORR (95% CI) 58% (48, 68) 35% (26, 45) Difference (95% CI) 23.6% (9.9, 36.4) p-Value§ 0.0008 Duration of Response‡ Median duration of response in months (range) 7.2 (2.4, 12.4+) 4.9 (2.0, 12.4+) At the protocol-specified final OS analysis, the median in the intravenous pembrolizumab in combination with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy arm was 17.1 months (95% CI: 14.4, 19.9) compared to 11.6 months (95% CI: 10.1, 13.7) in the placebo with carboplatin and either paclitaxel or paclitaxel protein-bound chemotherapy arm, with an HR of 0.71 (95% CI: 0.58, 0.88).
First-line treatment of metastatic NSCLC with PD-L1 expression (TPS ≥1%) as a single agent
KEYNOTE-042
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-042 (NCT02220894), a randomized, multicenter, open-label, active-controlled trial conducted in 1274 patients with Stage III NSCLC who were not candidates for surgical resection or definitive chemoradiation, or patients with metastatic NSCLC. Only patients whose tumors expressed PD-L1 (TPS ≥1%) by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx assay and who had not received prior systemic treatment for metastatic NSCLC were eligible. Patients with EGFR or ALK genomic tumor aberrations; autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of radiation in the thoracic region within the prior 26 weeks of initiation of study were ineligible. Randomization was stratified by ECOG PS (0 vs. 1), histology (squamous vs. nonsquamous), geographic region (East Asia vs. non-East Asia), and PD-L1 expression (TPS ≥50% vs. TPS 1 to 49%). Patients were randomized (1:1) to receive intravenous pembrolizumab 200 mg intravenously every 3 weeks or investigator's choice of either of the following platinum-containing chemotherapy regimens:
- Pemetrexed 500 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for a maximum of 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies;
- Paclitaxel 200 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for a maximum of 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies.
Treatment with intravenous pembrolizumab continued until RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined progression of disease, unacceptable toxicity, or a maximum of 24 months. Administration of intravenous pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator. Treatment with intravenous pembrolizumab could be reinitiated at the time of subsequent disease progression and administered for up to 12 months. Assessment of tumor status was performed every 9 weeks. The main efficacy outcome measure was OS in the subgroup of patients with TPS ≥50% NSCLC, the subgroup of patients with TPS ≥20% NSCLC, and the overall population with TPS ≥1% NSCLC. Additional efficacy outcome measures were PFS and ORR in the subgroup of patients with TPS ≥50% NSCLC, the subgroup of patients with TPS ≥20% NSCLC, and the overall population with TPS ≥1% NSCLC as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 63 years (range: 25 to 90), 45% age 65 or older; 71% male; and 64% White, 30% Asian, and 2% Black. Nineteen percent were Hispanic or Latino. Sixty-nine percent had ECOG PS of 1; 39% with squamous and 61% with nonsquamous histology; 87% had M1 disease and 13% had Stage IIIA (2%) or Stage IIIB (11%) and who were not candidates for surgical resection or definitive chemoradiation per investigator assessment; and 5% with treated brain metastases at baseline. Forty-seven percent of patients had TPS ≥50% NSCLC and 53% had TPS 1 to 49% NSCLC.
The trial demonstrated a statistically significant improvement in OS for patients (PD-L1 TPS ≥50%, TPS ≥20%, TPS ≥1%) randomized to intravenous pembrolizumab as compared with chemotherapy. Table 63 and Figure 7 summarize the efficacy results in the subgroup of patients with TPS ≥50% and in all randomized patients with TPS ≥1%.
Table 63: Efficacy Results of All Randomized Patients (TPS ≥1% and TPS ≥50%) in KEYNOTE-042 TPS ≥1% TPS ≥50% Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
ChemotherapyIntravenous Pembrolizumab
200 mg every 3 weeks
Chemotherapyn=637 n=637
n=299 n=300
- * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test; compared to a p-Value boundary of 0.0291
- ‡ Not evaluated for statistical significance as a result of the sequential testing procedure for the secondary endpoints
- § Not significant compared to a p-Value boundary of 0.0291
- ¶ Based on observed duration of response
OS Number of events (%) 371 (58%) 438 (69%) 157 (53%) 199 (66%) Median in months (95% CI) 16.7 (13.9, 19.7) 12.1 (11.3, 13.3) 20.0 (15.4, 24.9) 12.2 (10.4, 14.2) Hazard ratio* (95% CI) 0.81 (0.71, 0.93) 0.69 (0.56, 0.85) p-Value† 0.0036 0.0006 PFS Number of events (%) 507 (80%) 506 (79%) 221 (74%) 233 (78%) Median in months (95% CI) 5.4 (4.3, 6.2) 6.5 (6.3, 7.0) 6.9 (5.9, 9.0) 6.4 (6.1, 6.9) Hazard ratio*, ‡ (95% CI) 1.07
(0.94, 1.21)0.82
(0.68, 0.99)p-Value† -‡ NS§ Objective Response Rate ORR‡ (95% CI) 27% (24, 31) 27% (23, 30) 39% (33.9, 45.3) 32% (26.8, 37.6) Complete response rate 0.5% 0.5% 0.7% 0.3% Partial response rate 27% 26% 39% 32% Duration of Response % with duration ≥12 months¶ 47% 16% 42% 17% % with duration ≥18 months¶ 26% 6% 25% 5% The results of all efficacy outcome measures in the subgroup of patients with PD-L1 TPS ≥20% NSCLC were intermediate between the results of those with PD-L1 TPS ≥1% and those with PD-L1 TPS ≥50%. In a pre-specified exploratory subgroup analysis for patients with TPS 1-49% NSCLC, the median OS was 13.4 months (95% CI: 10.7, 18.2) for the pembrolizumab group and 12.1 months (95% CI: 11.0, 14.0) in the chemotherapy group, with an HR of 0.92 (95% CI: 0.77, 1.11).
Figure 7: Kaplan-Meier Curve for Overall Survival in all Randomized Patients in KEYNOTE-042 (TPS ≥1%) 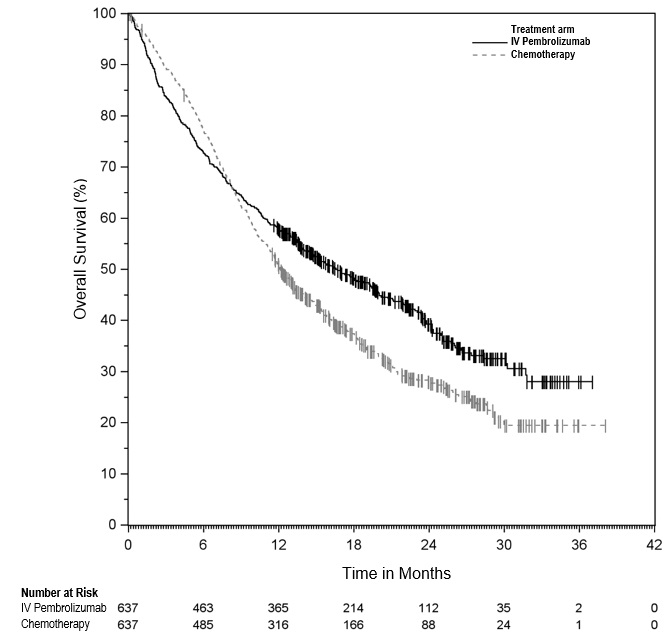
KEYNOTE-024
The efficacy of intravenous pembrolizumab was also investigated in KEYNOTE-024 (NCT02142738), a randomized, multicenter, open-label, active-controlled trial in 305 previously untreated patients with metastatic NSCLC. The study design was similar to that of KEYNOTE-042, except that only patients whose tumors had high PD-L1 expression (TPS of 50% or greater) by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx assay were eligible. Patients were randomized (1:1) to receive intravenous pembrolizumab 200 mg intravenously every 3 weeks or investigator's choice of any of the following platinum-containing chemotherapy regimens:
- Pemetrexed 500 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for 4 to 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies;
- Pemetrexed 500 mg/m2 every 3 weeks and cisplatin 75 mg/m2 every 3 weeks on Day 1 for 4 to 6 cycles followed by optional pemetrexed 500 mg/m2 every 3 weeks for patients with nonsquamous histologies;
- Gemcitabine 1250 mg/m2 on days 1 and 8 and cisplatin 75 mg/m2 every 3 weeks on Day 1 for 4 to 6 cycles;
- Gemcitabine 1250 mg/m2 on Days 1 and 8 and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for 4 to 6 cycles;
- Paclitaxel 200 mg/m2 every 3 weeks and carboplatin AUC 5 to 6 mg/mL/min every 3 weeks on Day 1 for 4 to 6 cycles followed by optional pemetrexed maintenance (for nonsquamous histologies).
Patients randomized to chemotherapy were offered intravenous pembrolizumab at the time of disease progression.
The main efficacy outcome measure was PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were OS and ORR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 65 years (range: 33 to 90), 54% age 65 or older; 61% male; 82% White and 15% Asian; 65% with ECOG PS of 1; 18% with squamous and 82% with nonsquamous histology and 9% with history of brain metastases. A total of 66 patients in the chemotherapy arm received intravenous pembrolizumab at the time of disease progression.
The trial demonstrated a statistically significant improvement in both PFS and OS for patients randomized to intravenous pembrolizumab as compared with chemotherapy. Table 64 and Figure 8 summarize the efficacy results for KEYNOTE-024.
Table 64: Efficacy Results in KEYNOTE-024 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeksChemotherapy n=154 n=151 NR = not reached - * Based on the stratified Cox proportional hazard model for the interim analysis
- † Based on the protocol-specified final OS analysis conducted at 169 events, which occurred 14 months after the interim analysis.
- ‡ p-Value is compared with 0.0118 of the allocated alpha for the interim analysis
PFS Number (%) of patients with event 73 (47%) 116 (77%) Median in months (95% CI) 10.3 (6.7, NR) 6.0 (4.2, 6.2) Hazard ratio* (95% CI) 0.50 (0.37, 0.68) p-Value (stratified log-rank) <0.001 OS Number (%) of patients with event 44 (29%) 64 (42%) Median in months (95% CI)† 30.0
(18.3, NR)14.2
(9.8, 19.0)Hazard ratio* (95% CI) 0.60 (0.41, 0.89) p-Value (stratified log-rank) 0.005‡ Objective Response Rate ORR (95% CI) 45% (37, 53) 28% (21, 36) Complete response rate 4% 1% Partial response rate 41% 27% p-Value (Miettinen-Nurminen) 0.001 Median duration of response in months (range) NR
(1.9+, 14.5+)6.3
(2.1+, 12.6+)Previously treated NSCLC with PD-L1 expression (TPS ≥1%)
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-010 (NCT01905657), a randomized, multicenter, open-label, active-controlled trial conducted in 1033 patients with metastatic NSCLC that had progressed following platinum-containing chemotherapy, and if appropriate, targeted therapy for EGFR or ALK genomic tumor aberrations. Eligible patients had PD-L1 expression TPS of 1% or greater by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx assay. Patients with autoimmune disease; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomization was stratified by tumor PD-L1 expression (PD-L1 expression TPS ≥50% vs. PD-L1 expression TPS=1-49%), ECOG PS (0 vs. 1), and geographic region (East Asia vs. non-East Asia). Patients were randomized (1:1:1) to receive intravenous pembrolizumab 2 mg/kg intravenously every 3 weeks, intravenous pembrolizumab 10 mg/kg intravenously every 3 weeks or docetaxel intravenously 75 mg/m2 every 3 weeks until unacceptable toxicity or disease progression. Patients randomized to intravenous pembrolizumab were permitted to continue until disease progression that was symptomatic, rapidly progressive, required urgent intervention, occurred with a decline in performance status, or confirmation of progression at 4 to 6 weeks with repeat imaging or for up to 24 months without disease progression. Assessment of tumor status was performed every 9 weeks. The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, in the subgroup of patients with TPS ≥50% and the overall population with TPS ≥1%. Additional efficacy outcome measures were ORR and DoR in the subgroup of patients with TPS ≥50% and the overall population with TPS ≥1%.
The study population characteristics were: median age of 63 years (range: 20 to 88), 42% age 65 or older; 61% male; 72% White and 21% Asian; 66% ECOG PS of 1; 43% with high PD-L1 tumor expression; 21% with squamous, 70% with nonsquamous, and 8% with mixed, other or unknown histology; 91% metastatic (M1) disease; 15% with history of brain metastases; and 8% and 1% with EGFR and ALK genomic aberrations, respectively. All patients had received prior therapy with a platinum-doublet regimen, 29% received two or more prior therapies for their metastatic disease.
Tables 65 and 66 and Figure 9 summarize efficacy results in the subgroup with TPS ≥50% population and in all patients, respectively.
Table 65: Efficacy Results of the Subgroup of Patients with TPS ≥50% in KEYNOTE-010 Endpoint Intravenous Pembrolizumab
2 mg/kg every 3 weeks
n=139Intravenous Pembrolizumab
10 mg/kg every 3 weeks
n=151Docetaxel
75 mg/m2 every 3 weeks
n=152NR = not reached - * Hazard ratio (intravenous pembrolizumab compared to docetaxel) based on the stratified Cox proportional hazard model
- † All responses were partial responses
OS Deaths (%) 58 (42%) 60 (40%) 86 (57%) Median in months (95% CI) 14.9 (10.4, NR) 17.3 (11.8, NR) 8.2 (6.4, 10.7) Hazard ratio* (95% CI) 0.54 (0.38, 0.77) 0.50 (0.36, 0.70) --- p-Value (stratified log-rank) <0.001 <0.001 --- PFS Events (%) 89 (64%) 97 (64%) 118 (78%) Median in months (95% CI) 5.2 (4.0, 6.5) 5.2 (4.1, 8.1) 4.1 (3.6, 4.3) Hazard ratio* (95% CI) 0.58 (0.43, 0.77) 0.59 (0.45, 0.78) --- p-Value (stratified log-rank) <0.001 <0.001 --- Objective Response Rate ORR† (95% CI) 30% (23, 39) 29% (22, 37) 8% (4, 13) p-Value (Miettinen-Nurminen) <0.001 <0.001 --- Median duration of response in months (range) NR
(0.7+, 16.8+)NR
(2.1+, 17.8+)8.1
(2.1+, 8.8+)Table 66: Efficacy Results of All Randomized Patients (TPS ≥1%) in KEYNOTE-010 Endpoint Intravenous Pembrolizumab
2 mg/kg every 3 weeks
n=344Intravenous Pembrolizumab
10 mg/kg every 3 weeks
n=346Docetaxel
75 mg/m2 every 3 weeks
n=343NR = not reached - * Hazard ratio (intravenous pembrolizumab compared to docetaxel) based on the stratified Cox proportional hazard model
- † All responses were partial responses
OS Deaths (%) 172 (50%) 156 (45%) 193 (56%) Median in months (95% CI) 10.4 (9.4, 11.9) 12.7 (10.0, 17.3) 8.5 (7.5, 9.8) Hazard ratio* (95% CI) 0.71 (0.58, 0.88) 0.61 (0.49, 0.75) --- p-Value (stratified log-rank) <0.001 <0.001 --- PFS Events (%) 266 (77%) 255 (74%) 257 (75%) Median in months (95% CI) 3.9 (3.1, 4.1) 4.0 (2.6, 4.3) 4.0 (3.1, 4.2) Hazard ratio* (95% CI) 0.88 (0.73, 1.04) 0.79 (0.66, 0.94) --- p-Value (stratified log-rank) 0.068 0.005 --- Objective Response Rate ORR† (95% CI) 18% (14, 23) 19% (15, 23) 9% (7, 13) p-Value (Miettinen-Nurminen) <0.001 <0.001 --- Median duration of response in months (range) NR
(0.7+, 20.1+)NR
(2.1+, 17.8+)6.2
(1.4+, 8.8+)Figure 9: Kaplan-Meier Curve for Overall Survival in all Randomized Patients in KEYNOTE-010 (TPS ≥1%) 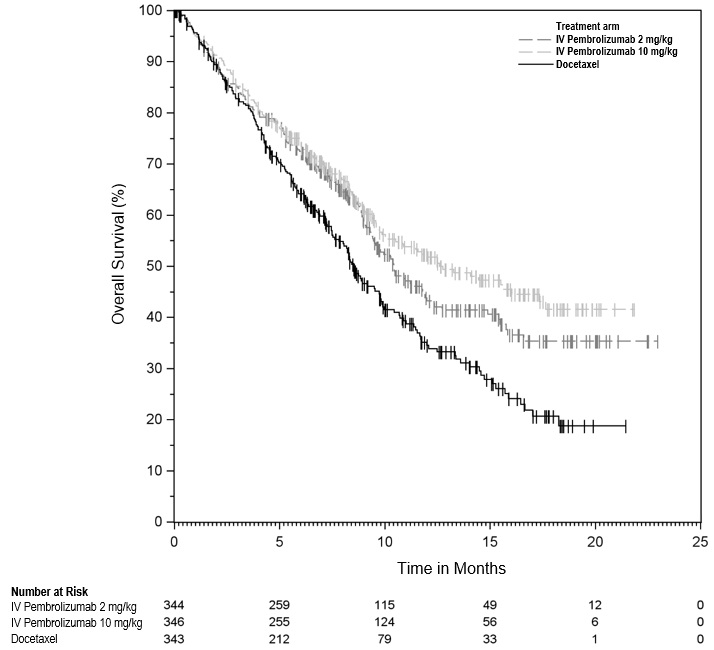
Neoadjuvant and adjuvant treatment of resectable NSCLC
The efficacy of intravenous pembrolizumab in combination with neoadjuvant chemotherapy followed by surgery and continued adjuvant treatment with intravenous pembrolizumab as a single agent was investigated in KEYNOTE-671 (NCT03425643), a multicenter, randomized, double-blind, placebo-controlled trial conducted in 797 patients with previously untreated and resectable Stage II, IIIA, or IIIB (N2) NSCLC by AJCC 8th edition. Patients were enrolled regardless of tumor PD-L1 expression. Patients with active autoimmune disease that required systemic therapy within 2 years of treatment, a medical condition that required immunosuppression, or a history of interstitial lung disease or pneumonitis that required steroids were ineligible. Randomization was stratified by stage (II vs. III), tumor PD-L1 expression (TPS ≥50% or <50%), histology (squamous vs. nonsquamous), and geographic region (East Asia vs. non-East Asia).
Patients were randomized (1:1) to one of the following treatment arms:
- Treatment Arm A: neoadjuvant intravenous pembrolizumab 200 mg on Day 1 in combination with cisplatin 75 mg/m2 and either pemetrexed 500 mg/m2 on Day 1 or gemcitabine 1000 mg/m2 on Days 1 and 8 of each 21-day cycle for up to 4 cycles. Within 4-12 weeks following surgery, intravenous pembrolizumab 200 mg was administered every 3 weeks for up to 13 cycles.
- Treatment Arm B: neoadjuvant placebo on Day 1 in combination with cisplatin 75 mg/m2 and either pemetrexed 500 mg/m2 on Day 1 or gemcitabine 1000 mg/m2 on Days 1 and 8 of each 21-day cycle for up to 4 cycles. Within 4-12 weeks following surgery, placebo was administered every 3 weeks for up to 13 cycles.
All study medications were administered via intravenous infusion. Treatment with intravenous pembrolizumab or placebo continued until completion of the treatment (17 cycles), disease progression that precluded definitive surgery, disease recurrence in the adjuvant phase, disease progression for those who did not undergo surgery or had incomplete resection and entered the adjuvant phase, or unacceptable toxicity. Assessment of tumor status was performed at baseline, Week 7, and Week 13 in the neoadjuvant phase and within 4 weeks prior to the start of the adjuvant phase. Following the start of the adjuvant phase, assessment of tumor status was performed every 16 weeks through the end of Year 3, and then every 6 months thereafter.
The trial was not designed to isolate the effect of intravenous pembrolizumab in each phase (neoadjuvant or adjuvant) of treatment.
The major efficacy outcome measures were OS and investigator-assessed event-free survival (EFS). Additional efficacy outcome measures were pathological complete response (pCR) rate and major pathological response (mPR) rate as assessed by blinded independent pathology review.
The study population characteristics were: median age of 64 years (range: 26 to 83); 45% age 65 or older and 7% age 75 or older; 71% male; 61% White, 31% Asian, 2% Black, 4% race not reported; 9% Hispanic or Latino; 63% ECOG PS of 0 and 37% ECOG PS of 1. Thirty percent had Stage II and 70% had Stage III disease; 33% had TPS ≥50% and 67% had TPS <50%; 43% had tumors with squamous histology and 57% had tumors with non-squamous histology; 31% were from the East Asian region.
Eighty-one percent of patients in the intravenous pembrolizumab in combination with platinum-containing chemotherapy arm received definitive surgery compared to 76% of patients in the placebo in combination with platinum-containing chemotherapy arm.
The trial demonstrated statistically significant improvements in OS and EFS for patients randomized to intravenous pembrolizumab in combination with platinum-containing chemotherapy followed by intravenous pembrolizumab as a single agent compared with patients randomized to placebo in combination with platinum-containing chemotherapy followed by placebo alone.
Table 67 and Figure 10 summarize the efficacy results for KEYNOTE-671.
Table 67: Efficacy Results in KEYNOTE-671 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
with
chemotherapy/Intravenous Pembrolizumab
n=397Placebo with
chemotherapy/Placebo
n=400NR = not reached - * Based on Kaplan-Meier estimates
- † Based on Cox regression model with treatment as a covariate stratified by stage, tumor PD-L1 expression, histology, and geographic region
- ‡ Based on stratified log-rank test
- § Compared to a two-sided p-Value boundary of 0.0109
- ¶ Compared to a two-sided p-Value boundary of 0.0092
OS Number of patients with event (%) 110 (28%) 144 (36%) Median in months* (95% CI) NR (NR, NR) 52.4 (45.7, NR) Hazard ratio† (95% CI) 0.72 (0.56, 0.93) p-Value‡,§ 0.0103 EFS Number of patients with event (%) 139 (35%) 205 (51%) Median in months* (95% CI) NR (34.1, NR) 17.0 (14.3, 22.0) Hazard ratio† (95% CI) 0.58 (0.46, 0.72) p-Value‡,¶ <0.0001 Figure 10: Kaplan-Meier Curve for Overall Survival in KEYNOTE-671 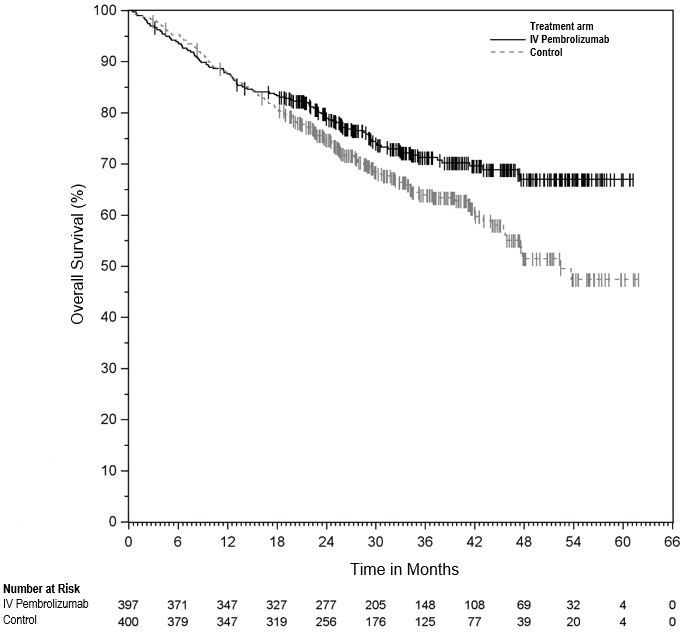
The trial demonstrated a statistically significant difference in pCR rate (18.1% vs. 4.0%; p<0.0001) and mPR rate (30.2% vs. 11.0%; p<0.0001).
Adjuvant treatment of resected NSCLC
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-091 (NCT02504372), a multicenter, randomized, triple-blind, placebo-controlled trial conducted in 1177 patients with completely resected Stage IB (T2a ≥4 cm), II, or IIIA NSCLC by AJCC 7th edition. Patients had not received neoadjuvant radiotherapy or chemotherapy. Adjuvant chemotherapy up to 4 cycles was optional. Patients were ineligible if they had active autoimmune disease, were on chronic immunosuppressive agents, or had a history of interstitial lung disease or pneumonitis. Randomization was stratified by stage (IB vs. II vs. IIIA), receipt of adjuvant chemotherapy (yes vs. no), PD-L1 status (TPS <1% [negative] vs. TPS 1-49% vs. TPS ≥50%), and geographic region (Western Europe vs. Eastern Europe vs. Asia vs. Rest of World). Patients were randomized (1:1) to receive intravenous pembrolizumab 200 mg or placebo intravenously every 3 weeks.
Treatment continued until RECIST v1.1-defined disease recurrence as determined by the investigator, unacceptable toxicity or up to one year. Tumor assessments were conducted every 12 weeks for the first year, then every 6 months for years 2 to 3, and then annually through year 5. After year 5, imaging was performed as per local standard of care. The major efficacy outcome measure was investigator-assessed disease-free survival (DFS). An additional efficacy outcome measure was OS.
Of 1177 patients randomized, 1010 (86%) received adjuvant platinum-based chemotherapy following resection. Among these 1010 patients, the median age was 64 years (range: 35 to 84), 49% age 65 or older; 68% male; 77% White, 18% Asian; 86% current or former smokers; and 39% with ECOG PS of 1. Eleven percent had Stage IB, 57% had Stage II, and 31% had Stage IIIA disease. Thirty-nine percent had PD-L1 TPS <1% [negative], 33% had TPS 1-49%, and 28% had TPS ≥50%. Fifty-two percent were from Western Europe, 20% from Eastern Europe, 17% from Asia, and 11% from Rest of World.
The trial met its primary endpoint, demonstrating a statistically significant improvement in DFS in the overall population for patients randomized to the intravenous pembrolizumab arm compared to patients randomized to the placebo arm. In an exploratory subgroup analysis of the 167 patients (14%) who did not receive adjuvant chemotherapy, the DFS HR was 1.25 (95% CI: 0.76, 2.05). OS results were not mature with only 42% of pre-specified OS events in the overall population.
Table 68 and Figure 11 summarize the efficacy results for KEYNOTE-091 in patients who received adjuvant chemotherapy.
Table 68: Efficacy Results in KEYNOTE-091 for Patients Who Received Adjuvant Chemotherapy Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=506Placebo
n=504NR = not reached - * Based on the unstratified univariate Cox regression model
DFS Number (%) of patients with event 177 (35%) 231 (46%) Median in months (95% CI) 58.7
(39.2, NR)34.9
(28.6, NR)Hazard ratio* (95% CI) 0.73 (0.60, 0.89) Figure 11: Kaplan-Meier Curve for Disease-Free Survival in KEYNOTE-091 for Patients Who Received Adjuvant Chemotherapy 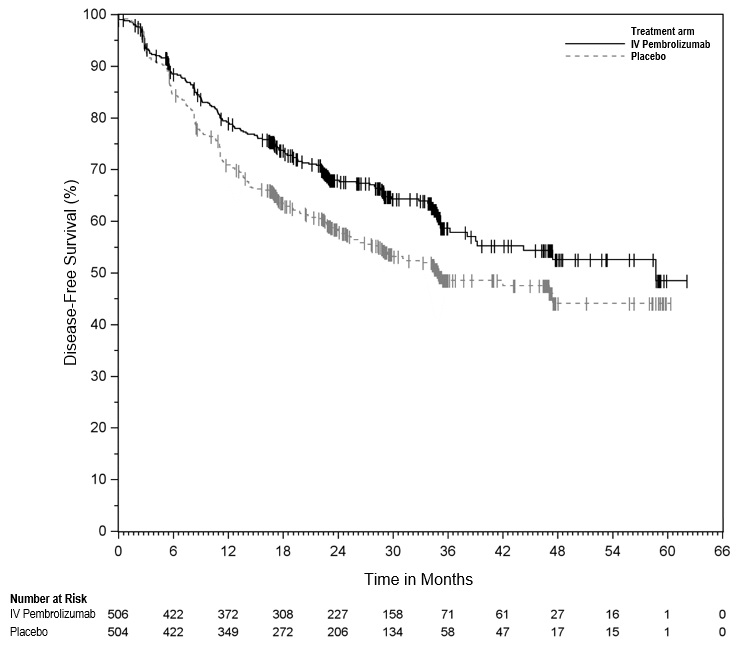
14.4 Malignant Pleural Mesothelioma
First-line treatment of unresectable advanced or metastatic malignant pleural mesothelioma (MPM) with pemetrexed and platinum chemotherapy
The efficacy of intravenous pembrolizumab in combination with pemetrexed and platinum chemotherapy was investigated in KEYNOTE-483 (NCT02784171), a multicenter, randomized, open-label, active-controlled trial that enrolled 440 patients with unresectable advanced or metastatic MPM and no prior systemic therapy for advanced/metastatic disease. Patients were enrolled regardless of tumor PD-L1 expression. Patients with autoimmune disease that required systemic therapy within 3 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by histological subtype (epithelioid vs. non-epithelioid). Patients were randomized (1:1) to one of the following treatment arms; all study medications were administered via intravenous infusion:
- Intravenous pembrolizumab 200 mg with pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 or carboplatin AUC 5-6 mg/mL/min on Day 1 of each 21-day cycle for up to 6 cycles, followed by intravenous pembrolizumab 200 mg every 3 weeks.
- Pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 or carboplatin AUC 5-6 mg/mL/min on Day 1 of each 21-day cycle for up to 6 cycles.
Treatment with intravenous pembrolizumab continued until disease progression as determined by the investigator according to modified RECIST 1.1 for mesothelioma (mRECIST), unacceptable toxicity, or a maximum of 24 months. Assessment of tumor status was performed every 6 weeks for 18 weeks, followed by every 12 weeks thereafter. The main efficacy outcome measure was OS. Additional efficacy outcome measures were PFS, ORR, and DoR, as assessed by BICR according to mRECIST.
The study population characteristics were: median age of 70 years (77% age 65 or older); 76% male; 79% White, 21% race not reported or unknown; 2% Hispanic or Latino; and 53% ECOG performance status of 1. Seventy-eight percent had epithelioid and 22% had non-epithelioid histology; 60% had tumors with PD-L1 CPS ≥1 and 30% had tumors with PD-L1 CPS <1.
The trial demonstrated a statistically significant improvement in OS, PFS, and ORR in patients randomized to intravenous pembrolizumab in combination with chemotherapy compared with patients randomized to chemotherapy alone. Table 69 and Figure 12 summarize the efficacy results for KEYNOTE-483.
Table 69: Efficacy Results in KEYNOTE-483 Endpoint Intravenous pembrolizumab
200 mg every 3 weeks
Pemetrexed
Platinum ChemotherapyPemetrexed
Platinum Chemotherapy(n=222) (n=218) - * Based on stratified Cox proportional hazard model
- † Based on stratified log-rank test
- ‡ Based on Miettinen and Nurminen method stratified by histological subtype at randomization (epithelioid vs. non-epithelioid)
- § Based on patients with a best overall response as confirmed complete or partial response; n=116 for patients in the intravenous pembrolizumab combination arm; n=63 for patients in the chemotherapy arm
OS Number (%) of patients with event 167 (75%) 175 (80%) Median in months (95% CI) 17.3 (14.4, 21.3) 16.1 (13.1, 18.2) Hazard ratio* (95% CI) 0.79 (0.64, 0.98) p-Value† 0.0162 PFS Number (%) of patients with event 190 (86%) 166 (76%) Median in months (95% CI) 7.1 (6.9, 8.1) 7.1 (6.8, 7.7) Hazard ratio* (95% CI) 0.80 (0.65, 0.99) p-Value† 0.0194 Objective Response Rate ORR % (95% CI) 52% (45.5, 59.0) 29% (23.0, 35.4) Complete responses 1 (0.5%) 0 (0%) Partial responses 115 (52%) 63 (29%) p-Value‡ <0.00001 Duration of Response§ Median in months (95% CI) 6.9 (5.8, 8.3) 6.8 (5.5, 8.5) Figure 12: Kaplan-Meier Curve for Overall Survival in KEYNOTE-483 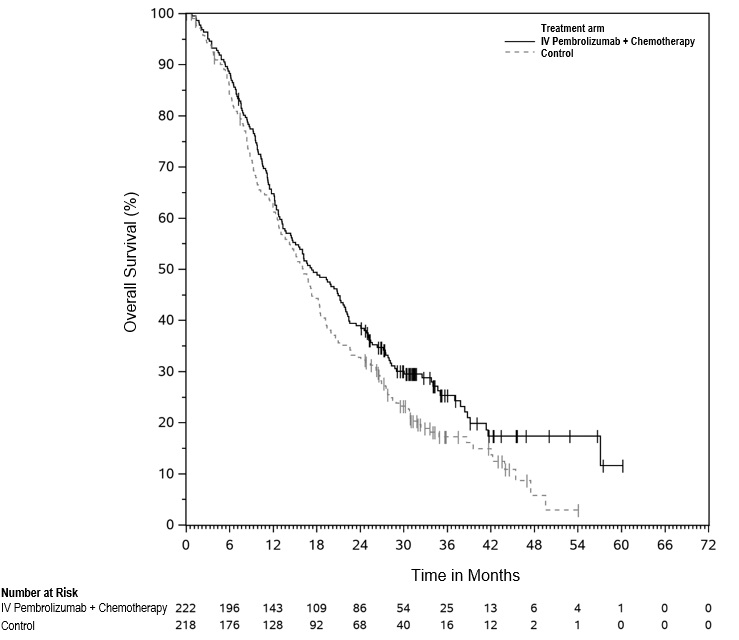
In a pre-specified exploratory analysis based on histology, in the subgroup of patients with epithelioid histology (n=345), the hazard ratio (HR) for OS was 0.89 (95% CI: 0.70, 1.13), with median OS of 19.8 months in intravenous pembrolizumab in combination with chemotherapy and 18.2 months in chemotherapy alone. In the subgroup of patients with non-epithelioid histology (n=95), the HR for OS was 0.57 (95% CI: 0.36, 0.89), with median OS of 12.3 months in intravenous pembrolizumab in combination with chemotherapy and 8.2 months in chemotherapy alone.
14.5 Head and Neck Squamous Cell Cancer
Neoadjuvant and Adjuvant Treatment of Locally Advanced HNSCC for Tumors Expressing PD-L1 (CPS ≥1)
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-689 (NCT02358031), a randomized, multicenter, open-label, active-controlled trial conducted in 714 patients with resectable locally advanced (Stage III-IVA) HNSCC [AJCC, 8th edition]. Patients with active autoimmune disease requiring systemic therapy within two years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by primary tumor site (oropharynx/oral cavity vs. larynx vs. hypopharynx), tumor stage (III vs. IVA) and PD-L1 status (TPS ≥ 50% vs. TPS<50%) according to the PD-L1 IHC 22C3 pharmDx assay.
Patients were randomized (1:1) to one of the following treatment arms:
- neoadjuvant intravenous pembrolizumab 200 mg for 2 cycles prior to surgical resection. Within 6 weeks following surgery, 3 cycles of adjuvant intravenous pembrolizumab 200 mg every 3 weeks in combination with radiotherapy (RT) with or without 3 cycles of cisplatin 100 mg/m2 every 3 weeks. This was followed by intravenous pembrolizumab 200 mg every 3 weeks for up to 12 cycles.
- no neoadjuvant treatment prior to surgery. Within 6 weeks following surgery, adjuvant RT with or without 3 cycles of concurrent cisplatin 100 mg/m2 every 3 weeks.
On both treatment arms, patients received cisplatin with adjuvant RT if high-risk pathological features (i.e., positive margins <1 mm or extranodal extension) were present at surgery.
Treatment with intravenous pembrolizumab continued until disease progression by RECIST v1.1 per BICR during the neoadjuvant phase that precluded surgery, local or metastatic recurrence during the adjuvant phase, completion of treatment, or unacceptable toxicity. Assessment of tumor status was performed prior to surgery at Week 6 in the neoadjuvant phase. Following the start of the adjuvant phase, assessment of tumor status was performed 12 weeks after end of RT with or without cisplatin treatment and then every 3 months until the end of year 3; then every 6 months thereafter up to the end of year 5.
The trial was not designed to isolate the effect of intravenous pembrolizumab in each phase (neoadjuvant or adjuvant) of treatment.
The major efficacy outcome measure was event-free survival (EFS) by BICR defined as the time from randomization to the first occurrence of any of the following events: progression of disease that precludes definitive surgery, local or distant disease progression or recurrence, or death due to any cause. Additional efficacy outcome measures were major pathological response (mPR) as assessed by BIPR, and overall survival (OS).
The demographic and baseline characteristics in the 682 patients with PD-L1 expression of CPS ≥1 were: median age of 60 years (range: 22 to 87), 33% age 65 or older; 79% male; 78% White, 13% Asian and 2.5% Black or African American, 14% were Hispanic or Latino; 43% had ECOG PS of 1, and 79% were former/current smokers. Four percent of patients’ tumors were HPV-positive, and 26% had Stage III disease, 74% had Stage IVA disease. Sixty-eight percent of patients’ tumors had PD-L1 expression of CPS ≥10.
Eighty-eight percent of patients received definitive surgery in both the intravenous pembrolizumab and the SOC arm.
Seventy-six percent of patients in the intravenous pembrolizumab arm and 78% of patients in the SOC arm started the radiation phase of treatment. In the intravenous pembrolizumab arm, 35% of patients received intravenous pembrolizumab and cisplatin with concurrent RT, 57% of patients received intravenous pembrolizumab alone with concurrent RT, 3% of patients received cisplatin alone with concurrent RT, 5% of patients received RT alone and one patient (0.4%) received intravenous pembrolizumab alone without concurrent RT. On the SoC arm, 52% of patients received cisplatin with concurrent RT while 48% patients received RT alone.
The trial demonstrated a statistically significant improvement in EFS for patients randomized to the intravenous pembrolizumab arm compared to those randomized to the standard of care (SOC) arm at the first pre-specified interim analysis. Table 70 and Figure 13 summarize efficacy results in KEYNOTE-689 for patients with HNSCC CPS ≥1.
Table 70: Efficacy Results for Perioperative Intravenous Pembrolizumab with adjuvant RT with or without cisplatin in Patients with HNSCC CPS ≥1 in KEYNOTE-689 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
with
RT with or without cisplatin
n=347RT with or without cisplatin
n=335NR = not reached - * From product-limit (Kaplan-Meier) method for censored data.
- † Based on Cox regression model with Efron’s method of tie handling with treatment as a covariate stratified by primary tumor site and tumor stage.
- ‡ One-sided p-value based on log-rank test stratified by primary tumor site and tumor stage. Compared to a one-sided p-value boundary of 0.0124
EFS Number of events, n (%) 128 (37%) 156 (47%) Median in months* (95% CI) 59.7 (37.9, NR) 29.6 (19.5, 41.9) Hazard ratio† (95% CI) 0.70 (0.55, 0.89) p-Value‡, 0.00140 Figure 13: Kaplan-Meier Curve for Event-free Survival for Intravenous Pembrolizumab in Patients with HNSCC CPS ≥1 in KEYNOTE-689 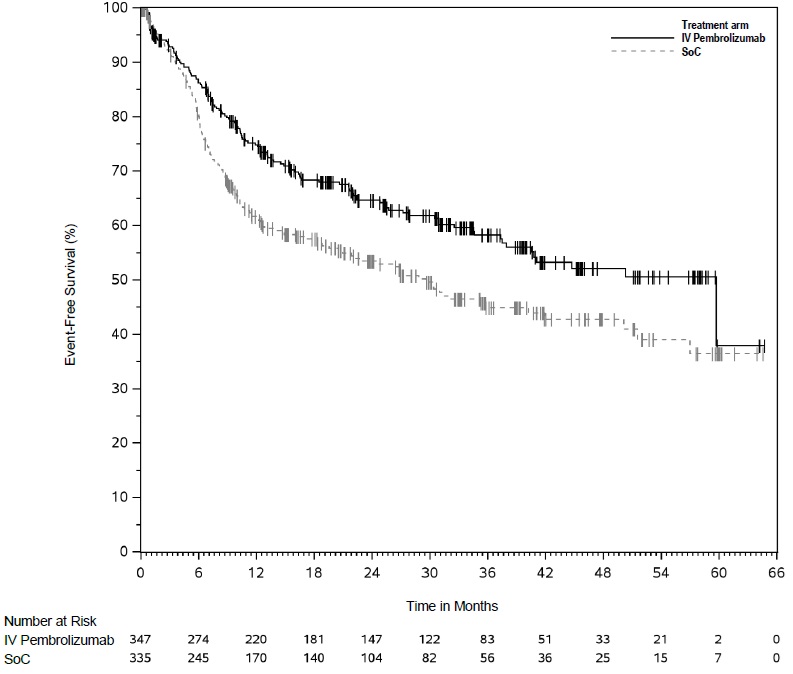
While OS results were not mature at this interim analysis, with 76% of pre-specified OS events in the CPS ≥1 population, no trend towards a detriment was observed.
Hypopharyngeal Tumors
In an exploratory subgroup analysis of patients with PD-L1-positive (CPS ≥1) hypopharyngeal tumors who were randomized (n=51), the EFS HR was 2.28 (95% CI: 0.79, 6.56). Among these patients, 23 patients in the intravenous pembrolizumab arm received surgery, of which 17 patients (74%) had R0 resections. On the SOC arm, 23 patients received surgery, of which 20 (87%) had R0 resections.
Oral Cavity, Oropharyngeal and Laryngeal Tumors
In an exploratory subgroup analysis of patients with PD-L1 positive (CPS ≥1) oral cavity, oropharyngeal and laryngeal tumors who received surgery (n=555), 91% of patients on the intravenous pembrolizumab arm had R0 resections while 85% of patients on the SOC arm had R0 resections.
First-line treatment of metastatic or unresectable, recurrent HNSCC
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-048 (NCT02358031), a randomized, multicenter, open-label, active-controlled trial conducted in 882 patients with metastatic HNSCC who had not previously received systemic therapy for metastatic disease or with recurrent disease who were considered incurable by local therapies. Patients with active autoimmune disease that required systemic therapy within two years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by tumor PD-L1 expression (TPS ≥50% or <50%) according to the PD-L1 IHC 22C3 pharmDx assay, HPV status according to p16 IHC (positive or negative), and ECOG PS (0 vs. 1). Patients were randomized 1:1:1 to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg intravenously every 3 weeks
- Intravenous pembrolizumab 200 mg intravenously every 3 weeks, carboplatin AUC 5 mg/mL/min intravenously every 3 weeks or cisplatin 100 mg/m2 intravenously every 3 weeks, and FU 1000 mg/m2/day as a continuous intravenous infusion over 96 hours every 3 weeks (maximum of 6 cycles of platinum and FU)
- Cetuximab 400 mg/m2 intravenously as the initial dose then 250 mg/m2 intravenously once weekly, carboplatin AUC 5 mg/mL/min intravenously every 3 weeks or cisplatin 100 mg/m2 intravenously every 3 weeks, and FU 1000 mg/m2/day as a continuous intravenous infusion over 96 hours every 3 weeks (maximum of 6 cycles of platinum and FU)
Treatment with intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Administration of intravenous pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at Week 9 and then every 6 weeks for the first year, followed by every 9 weeks through 24 months. A retrospective re-classification of patients' tumor PD-L1 status according to CPS using the PD-L1 IHC 22C3 pharmDx assay was conducted using the tumor specimens used for randomization.
The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ) sequentially tested in the subgroup of patients with CPS ≥20, the subgroup of patients with CPS ≥1, and the overall population.
The study population characteristics were: median age of 61 years (range: 20 to 94), 36% age 65 or older; 83% male; 73% White, 20% Asian and 2.4% Black; 61% had ECOG PS of 1; and 79% were former/current smokers. Twenty-two percent of patients' tumors were HPV-positive, 23% had PD-L1 TPS ≥50%, and 95% had Stage IV disease (Stage IVA 19%, Stage IVB 6%, and Stage IVC 70%). Eighty-five percent of patients' tumors had PD-L1 expression of CPS ≥1 and 43% had CPS ≥20.
The trial demonstrated a statistically significant improvement in OS for patients randomized to intravenous pembrolizumab in combination with chemotherapy compared to those randomized to cetuximab in combination with chemotherapy at a pre-specified interim analysis in the overall population. Table 71 and Figure 14 summarize efficacy results for intravenous pembrolizumab in combination with chemotherapy.
Table 71: Efficacy Results* for Intravenous Pembrolizumab plus Platinum/Fluorouracil in KEYNOTE-048 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
Platinum
FUCetuximab
Platinum
FUn=281 n=278 - * Results at a pre-specified interim analysis
- † Based on the stratified Cox proportional hazard model
- ‡ Based on stratified log-rank test
- § Response: Best objective response as confirmed complete response or partial response
OS Number (%) of patients with event 197 (70%) 223 (80%) Median in months (95% CI) 13.0 (10.9, 14.7) 10.7 (9.3, 11.7) Hazard ratio† (95% CI) 0.77 (0.63, 0.93) p-Value‡ 0.0067 PFS Number of patients with event (%) 244 (87%) 253 (91%) Median in months (95% CI) 4.9 (4.7, 6.0) 5.1 (4.9, 6.0) Hazard ratio† (95% CI) 0.92 (0.77, 1.10) p-Value‡ 0.3394 Objective Response Rate ORR§ (95% CI) 36% (30.0, 41.5) 36% (30.7, 42.3) Complete response rate 6% 3% Partial response rate 30% 33% Duration of Response Median in months (range) 6.7 (1.6+, 30.4+) 4.3 (1.2+, 27.9+) At the pre-specified final OS analysis for the ITT population, the hazard ratio was 0.72 (95% CI: 0.60, 0.87). In addition, KEYNOTE-048 demonstrated a statistically significant improvement in OS for the subgroups of patients with PD-L1 CPS ≥1 (HR=0.65, 95% CI: 0.53, 0.80) and CPS ≥20 (HR=0.60, 95% CI: 0.45, 0.82).
Figure 14: Kaplan-Meier Curve for Overall Survival for Intravenous Pembrolizumab plus Platinum/Fluorouracil in KEYNOTE-048* - * At the time of the protocol-specified final analysis.
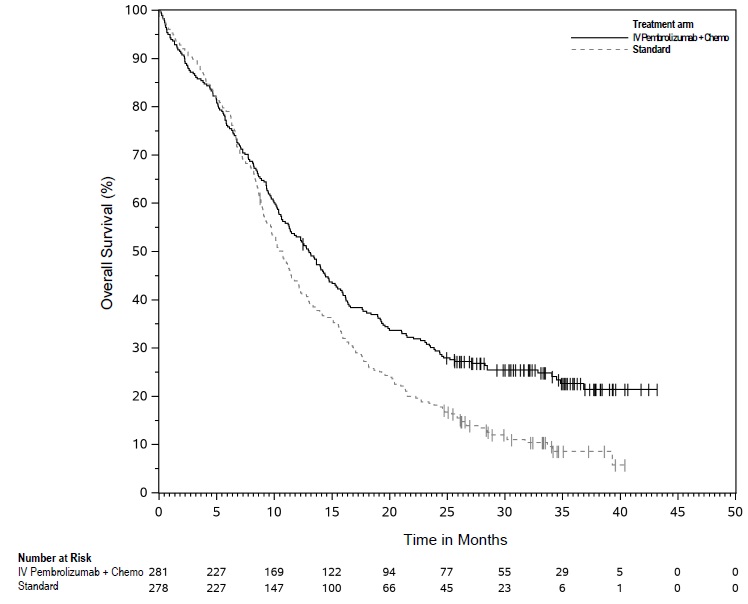
First-line treatment of metastatic or unresectable, recurrent HNSCC for Tumors Expressing PD-L1 (CPS ≥1) as a single agent
The trial also demonstrated a statistically significant improvement in OS for the subgroup of patients with PD-L1 CPS ≥1 randomized to intravenous pembrolizumab as a single agent compared to those randomized to cetuximab in combination with chemotherapy at a pre-specified interim analysis. At the time of the interim and final analyses, there was no significant difference in OS between the intravenous pembrolizumab single agent arm and the control arm for the overall population.
Table 72 summarizes efficacy results for intravenous pembrolizumab as a single agent in the subgroups of patients with CPS ≥1 HNSCC and CPS ≥20 HNSCC. Figure 15 summarizes the OS results in the subgroup of patients with CPS ≥1 HNSCC.
Table 72: Efficacy Results* for Intravenous Pembrolizumab as a Single Agent in KEYNOTE-048 (CPS ≥1 and CPS ≥20) Endpoint CPS ≥1 CPS ≥20 Intravenous Pembrolizumab
200 mg every 3 weeksCetuximab
Platinum
FUIntravenous Pembrolizumab
200 mg every 3 weeksCetuximab
Platinum
FUn=257 n=255 n=133 n=122 - * Results at a pre-specified interim analysis
- † Based on the stratified Cox proportional hazard model
- ‡ Based on a stratified log-rank test
- § Response: Best objective response as confirmed complete response or partial response
OS Number of events (%) 177 (69%) 206 (81%) 82 (62%) 95 (78%) Median in months (95% CI) 12.3 (10.8, 14.9) 10.3 (9.0, 11.5) 14.9 (11.6, 21.5) 10.7 (8.8, 12.8) Hazard ratio† (95% CI) 0.78 (0.64, 0.96) 0.61 (0.45, 0.83) p-Value‡ 0.0171 0.0015 PFS Number of events (%) 225 (88%) 231 (91%) 113 (85%) 111 (91%) Median in months (95% CI) 3.2 (2.2, 3.4) 5.0 (4.8, 5.8) 3.4 (3.2, 3.8) 5.0 (4.8, 6.2) Hazard ratio† (95% CI) 1.15 (0.95, 1.38) 0.97 (0.74, 1.27) Objective Response Rate ORR§ (95% CI) 19% (14.5, 24.4) 35% (29.1, 41.1) 23% (16.4, 31.4) 36% (27.6, 45.3) Complete response rate 5% 3% 8% 3% Partial response rate 14% 32% 16% 33% Duration of Response Median in months (range) 20.9 (1.5+, 34.8+) 4.5 (1.2+, 28.6+) 20.9 (2.7, 34.8+) 4.2 (1.2+, 22.3+) At the pre-specified final OS analysis comparing intravenous pembrolizumab as a single agent to cetuximab in combination with chemotherapy, the hazard ratio for the subgroup of patients with CPS ≥1 was 0.74 (95% CI: 0.61, 0.90) and the hazard ratio for the subgroup of patients with CPS ≥20 was 0.58 (95% CI: 0.44, 0.78).
In an exploratory subgroup analysis for patients with CPS 1-19 HNSCC at the time of the pre-specified final OS analysis, the median OS was 10.8 months (95% CI: 9.0, 12.6) for intravenous pembrolizumab as a single agent and 10.1 months (95% CI: 8.7, 12.1) for cetuximab in combination with chemotherapy, with an HR of 0.86 (95% CI: 0.66, 1.12).
Previously treated recurrent or metastatic HNSCC
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-012 (NCT01848834), a multicenter, non-randomized, open-label, multi-cohort study that enrolled 174 patients with recurrent or metastatic HNSCC who had disease progression on or after platinum-containing chemotherapy administered for recurrent or metastatic HNSCC or following platinum-containing chemotherapy administered as part of induction, concurrent, or adjuvant therapy. Patients with active autoimmune disease, a medical condition that required immunosuppression, evidence of interstitial lung disease, or ECOG PS ≥2 were ineligible.
Patients received intravenous pembrolizumab 10 mg/kg every 2 weeks (n=53) or 200 mg every 3 weeks (n=121) until unacceptable toxicity or disease progression that was symptomatic, was rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at least 4 weeks later with repeat imaging. Patients without disease progression were treated for up to 24 months. Treatment with pembrolizumab could be reinitiated for subsequent disease progression and administered for up to 1 additional year. Assessment of tumor status was performed every 8 weeks. The major efficacy outcome measures were ORR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR, and DoR.
The study population characteristics were median age of 60 years, 32% age 65 or older; 82% male; 75% White, 16% Asian, and 6% Black; 87% had M1 disease; 33% had HPV positive tumors; 63% had prior cetuximab; 29% had an ECOG PS of 0 and 71% had an ECOG PS of 1; and the median number of prior lines of therapy administered for the treatment of HNSCC was 2.
The ORR was 16% (95% CI: 11, 22) with a complete response rate of 5%. The median follow-up time was 8.9 months. Among the 28 responding patients, the median DoR had not been reached (range: 2.4+ to 27.7+ months), with 23 patients having responses of 6 months or longer. The ORR and DoR were similar irrespective of dosage regimen (10 mg/kg every 2 weeks or 200 mg every 3 weeks) or HPV status.
14.6 Urothelial Cancer
In Combination with Enfortumab Vedotin for the Treatment of Patients with Urothelial Cancer
The efficacy of intravenous pembrolizumab in combination with enfortumab vedotin was evaluated in KEYNOTE-A39 (NCT04223856), an open-label, randomized, multicenter trial that enrolled 886 patients with locally advanced or metastatic urothelial cancer who received no prior systemic therapy for locally advanced or metastatic disease. Patients with active CNS metastases, ongoing sensory or motor neuropathy Grade ≥2, or uncontrolled diabetes defined as hemoglobin A1C (HbA1c) ≥8% or HbA1c ≥7% with associated diabetes symptoms were excluded.
Patients were randomized 1:1 to receive either:
- Intravenous pembrolizumab 200 mg over 30 minutes on Day 1 and enfortumab vedotin 1.25 mg/kg on Days 1 and 8 of each 21-day cycle. Intravenous pembrolizumab was given approximately 30 minutes after enfortumab vedotin. Treatment was continued until disease progression or unacceptable toxicity. In the absence of disease progression or unacceptable toxicity, intravenous pembrolizumab was continued for up to 2 years.
- Gemcitabine 1000 mg/m2 on Days 1 and 8 of a 21-day cycle with cisplatin 70 mg/m2 or carboplatin (AUC = 4.5 or 5) on Day 1 of a 21-day cycle. Treatment was continued until disease progression or unacceptable toxicity for up to 6 cycles.
Randomization was stratified by cisplatin eligibility, PD-L1 expression, and presence of liver metastases.
The median age was 69 years (range: 22 to 91); 77% were male; 67% were White, 22% were Asian, 1% were Black or African American, and 10% were unknown or other; 12% were Hispanic or Latino. Patients had a baseline ECOG performance status of 0 (49%), 1 (47%), or 2 (3%). Forty-seven percent of patients had a documented baseline HbA1c of <5.7%. At baseline, 95% of patients had metastatic urothelial cancer, including 72% with visceral and 22% with liver metastases, and 5% had locally advanced urothelial cancer. Eighty-five percent of patients had urothelial carcinoma (UC) histology including 6% with UC mixed squamous differentiation and 2% with UC mixed other histologic variants. Forty-six percent of patients were considered cisplatin-ineligible and 54% were considered cisplatin-eligible at time of randomization.
The major efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1. Additional efficacy outcome measures included ORR as assessed by BICR.
The trial demonstrated statistically significant improvements in OS, PFS, and ORR for patients randomized to intravenous pembrolizumab in combination with enfortumab vedotin as compared to platinum-based chemotherapy. Efficacy results were consistent across all stratified patient subgroups.
Table 73 and Figures 16 and 17 summarize the efficacy results for KEYNOTE-A39.
Table 73: Efficacy Results in KEYNOTE-A39 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks in combination with Enfortumab Vedotin
n=442Cisplatin or Carboplatin with Gemcitabine
n=444NR = not reached - * Based on the stratified Cox proportional hazard regression model
- † Two-sided p-Value based on stratified log-rank test
- ‡ Includes only patients with measurable disease at baseline (n=437 for intravenous pembrolizumab in combination with enfortumab vedotin, n=441 for chemotherapy).
- § Based on patients with a best overall response as confirmed complete or partial response
- ¶ Two-sided p-Value based on Cochran-Mantel-Haenszel test stratified by PD-L1 expression, cisplatin eligibility and liver metastases
OS Number (%) of patients with event 133 (30%) 226 (51%) Median in months (95% CI) 31.5 (25.4, NR) 16.1 (13.9, 18.3) Hazard ratio* (95% CI) 0.47 (0.38, 0.58) p-Value† <0.0001 PFS Number (%) of patients with event 223 (50%) 307 (69%) Median in months (95% CI) 12.5 (10.4, 16.6) 6.3 (6.2, 6.5) Hazard ratio* (95% CI) 0.45 (0.38, 0.54) p-Value† <0.0001 Confirmed Objective Response Rate‡ ORR§ % (95% CI) 68% (63, 72) 44% (40, 49) p-Value¶ <0.0001 Complete response 29% 12% Partial response 39% 32% Figure 16: Kaplan-Meier Curve for Overall Survival in KEYNOTE-A39 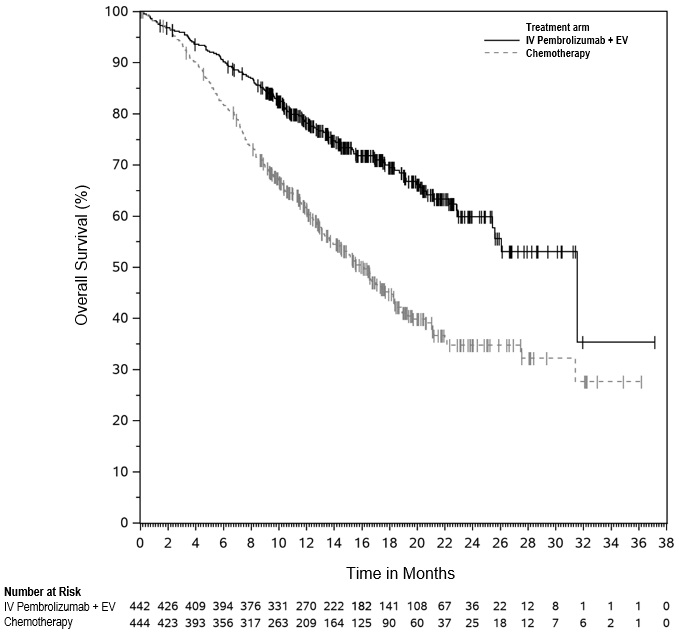
Figure 17: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-A39 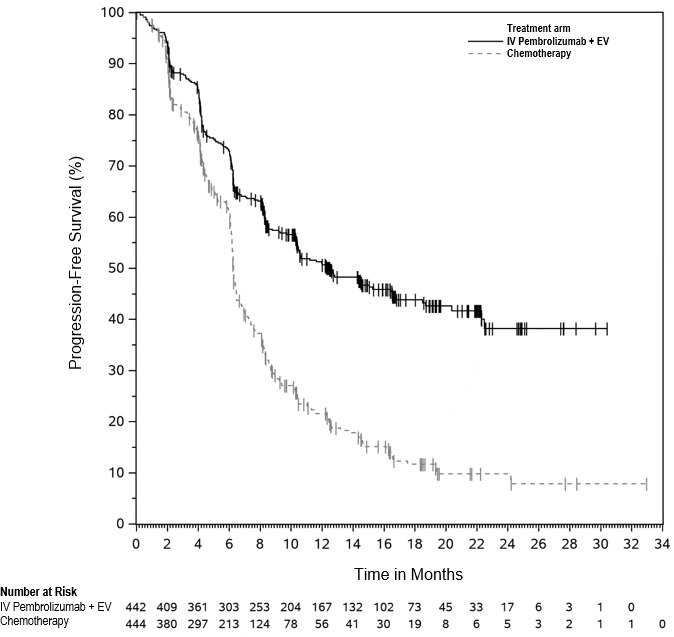
In Combination with Enfortumab Vedotin for the Treatment of Cisplatin-Ineligible Patients with Urothelial Cancer
The efficacy of intravenous pembrolizumab in combination with enfortumab vedotin was evaluated in KEYNOTE-869 (NCT03288545), an open-label, multi-cohort (dose escalation cohort, Cohort A, Cohort K) study in patients with locally advanced or metastatic urothelial cancer who were ineligible for cisplatin-containing chemotherapy and received no prior systemic therapy for locally advanced or metastatic disease. Patients with active CNS metastases, ongoing sensory or motor neuropathy Grade ≥2, or uncontrolled diabetes defined as hemoglobin A1C (HbA1c) ≥8% or HbA1c ≥7% with associated diabetes symptoms were excluded from participating in the study.
Patients in the dose escalation cohort (n=5), Cohort A (n=40), and Cohort K (n=76) received enfortumab vedotin 1.25 mg/kg as an IV infusion over 30 minutes on Days 1 and 8 of a 21-day cycle followed by intravenous pembrolizumab 200 mg as an IV infusion on Day 1 of a 21-day cycle approximately 30 minutes after enfortumab vedotin. Patients were treated until disease progression or unacceptable toxicity.
A total of 121 patients received intravenous pembrolizumab in combination with enfortumab vedotin. The median age was 71 years (range: 51 to 91); 74% were male; 85% were White, 5% were Black, 4% were Asian and 6% were other, unknown or not reported. Ten percent of patients were Hispanic or Latino. Forty-five percent of patients had an ECOG performance status of 1 and 15% had an ECOG performance status of 2. Forty-seven percent of patients had a documented baseline HbA1c of <5.7%. Reasons for cisplatin-ineligibility included: 60% with baseline creatinine clearance of 30-59 mL/min, 10% with ECOG PS of 2, 13% with Grade 2 or greater hearing loss, and 16% with more than one cisplatin-ineligibility criteria.
At baseline, 97.5% of patients had metastatic urothelial cancer and 2.5% of patients had locally advanced urothelial cancer. Thirty-seven percent of patients had upper tract disease. Eighty-four percent of patients had visceral metastasis at baseline, including 22% with liver metastases. Thirty-nine percent of patients had TCC histology; 13% had TCC with squamous differentiation, and 48% had TCC with other histologic variants.
The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1.
The median follow-up time for the dose escalation cohort + Cohort A was 44.7 months (range 0.7 to 52.4) and for Cohort K was 14.8 months (range: 0.6 to 26.2).
Efficacy results are presented in Table 74 below.
Table 74: Efficacy Results in KEYNOTE-869, Combined Dose Escalation Cohort, Cohort A, and Cohort K Endpoint Intravenous Pembrolizumab in combination with Enfortumab Vedotin
n=121Confirmed ORR (95% CI) 68% (58.7, 76.0) Complete response rate 12% Partial response rate 55% The median duration of response for the dose escalation cohort + Cohort A was 22.1 months (range: 1.0+ to 46.3+) and for Cohort K was not reached (range: 1.2 to 24.1+).
Platinum-Ineligible Patients with Urothelial Carcinoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-052 (NCT02335424), a multicenter, open-label, single-arm trial in 370 patients with locally advanced or metastatic urothelial carcinoma who had one or more comorbidities, including patients who were not eligible for any platinum-containing chemotherapy. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression. Patients received intravenous pembrolizumab 200 mg every 3 weeks until unacceptable toxicity or disease progression. Patients with initial radiographic disease progression could receive additional doses of treatment during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status. Patients without disease progression could be treated for up to 24 months. Tumor response assessments were performed at 9 weeks after the first dose, then every 6 weeks for the first year, and then every 12 weeks thereafter. The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study population characteristics were: median age of 74 years; 77% male; and 89% White. Eighty-seven percent had M1 disease, and 13% had M0 disease. Eighty-one percent had a primary tumor in the lower tract, and 19% of patients had a primary tumor in the upper tract. Eighty-five percent of patients had visceral metastases, including 21% with liver metastases. Fifty percent of patients had baseline creatinine clearance of <60 mL/min, 32% had ECOG PS of 2, 9% had ECOG PS of 2 and baseline creatinine clearance of <60 mL/min, and 9% had one or more of Class III heart failure, Grade 2 or greater peripheral neuropathy, and Grade 2 or greater hearing loss. Ninety percent of patients were treatment naïve, and 10% received prior adjuvant or neoadjuvant platinum-based chemotherapy.
The median follow-up time for 370 patients treated with intravenous pembrolizumab was 11.4 months (range 0.1 to 63.8 months). Efficacy results are summarized in Table 75.
Table 75: Efficacy Results in KEYNOTE-052 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeksAll Subjects
n=370+ Denotes ongoing response Objective Response Rate ORR (95% CI) 29% (24, 34) Complete response rate 10% Partial response rate 20% Duration of Response Median in months (range) 33.4
(1.4+, 60.7+)Platinum-Eligible Patients with Previously Untreated Urothelial Carcinoma
The efficacy of intravenous pembrolizumab for the first-line treatment of platinum-eligible patients with locally advanced or metastatic urothelial carcinoma was investigated in KEYNOTE-361 (NCT02853305), a multicenter, randomized, open-label, active-controlled study in 1010 previously untreated patients. The safety and efficacy of intravenous pembrolizumab in combination with platinum-based chemotherapy for previously untreated patients with locally advanced or metastatic urothelial carcinoma has not been established.
The study compared intravenous pembrolizumab with or without platinum-based chemotherapy (i.e., cisplatin or carboplatin with gemcitabine) to platinum-based chemotherapy alone. Among the patients receiving intravenous pembrolizumab plus platinum-based chemotherapy, 44% received cisplatin and 56% received carboplatin.
The study did not meet its major efficacy outcome measures of improved PFS or OS in the intravenous pembrolizumab plus chemotherapy arm compared to the chemotherapy-alone arm. Additional efficacy endpoints, including improvement of OS in the intravenous pembrolizumab monotherapy arm, could not be formally tested.
Previously Treated Urothelial Carcinoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-045 (NCT02256436), a multicenter, randomized (1:1), active-controlled trial in 542 patients with locally advanced or metastatic urothelial carcinoma with disease progression on or after platinum-containing chemotherapy. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression.
Patients were randomized to receive either intravenous pembrolizumab 200 mg every 3 weeks (n=270) or investigator's choice of any of the following chemotherapy regimens all given intravenously every 3 weeks (n=272): paclitaxel 175 mg/m2 (n=90), docetaxel 75 mg/m2 (n=92), or vinflunine 320 mg/m2 (n=90). Treatment continued until unacceptable toxicity or disease progression. Patients with initial radiographic disease progression could receive additional doses of treatment during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status. Patients without disease progression could be treated for up to 24 months. Assessment of tumor status was performed at 9 weeks after randomization, then every 6 weeks through the first year, followed by every 12 weeks thereafter. The major efficacy outcomes were OS and PFS as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were ORR as assessed by BICR per RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and DoR.
The study population characteristics were: median age of 66 years (range: 26 to 88), 58% age 65 or older; 74% male; 72% White and 23% Asian; 42% ECOG PS of 0 and 56% ECOG PS of 1; and 96% M1 disease and 4% M0 disease. Eighty-seven percent of patients had visceral metastases, including 34% with liver metastases. Eighty-six percent had a primary tumor in the lower tract and 14% had a primary tumor in the upper tract. Fifteen percent of patients had disease progression following prior platinum-containing neoadjuvant or adjuvant chemotherapy. Twenty-one percent had received 2 or more prior systemic regimens in the metastatic setting. Seventy-six percent of patients received prior cisplatin, 23% had prior carboplatin, and 1% were treated with other platinum-based regimens.
The study demonstrated statistically significant improvements in OS and ORR for patients randomized to intravenous pembrolizumab as compared to chemotherapy. There was no statistically significant difference between intravenous pembrolizumab and chemotherapy with respect to PFS. The median follow-up time for this trial was 9.0 months (range: 0.2 to 20.8 months). Table 76 and Figure 18 summarize the efficacy results for KEYNOTE-045.
Table 76: Efficacy Results in KEYNOTE-045 Intravenous Pembrolizumab
200 mg every 3 weeksChemotherapy n=270 n=272 + Denotes ongoing response
NR = not reached- * Hazard ratio (intravenous pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
OS Deaths (%) 155 (57%) 179 (66%) Median in months (95% CI) 10.3 (8.0, 11.8) 7.4 (6.1, 8.3) Hazard ratio* (95% CI) 0.73 (0.59, 0.91) p-Value (stratified log-rank) 0.004 PFS by BICR Events (%) 218 (81%) 219 (81%) Median in months (95% CI) 2.1 (2.0, 2.2) 3.3 (2.3, 3.5) Hazard ratio* (95% CI) 0.98 (0.81, 1.19) p-Value (stratified log-rank) 0.833 Objective Response Rate ORR (95% CI) 21% (16, 27) 11% (8, 16) Complete response rate 7% 3% Partial response rate 14% 8% p-Value (Miettinen-Nurminen) 0.002 Median duration of response in months (range) NR
(1.6+, 15.6+)4.3
(1.4+, 15.4+)Figure 18: Kaplan-Meier Curve for Overall Survival in KEYNOTE-045 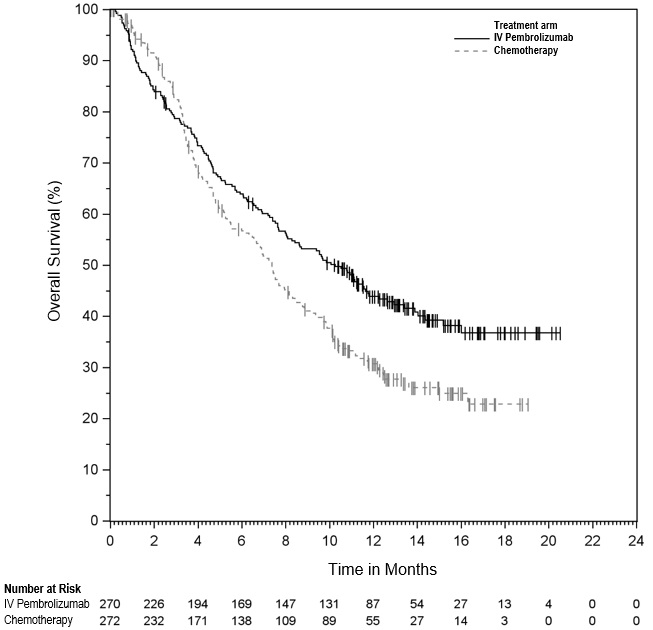
Neoadjuvant and Adjuvant Treatment of Cisplatin-Ineligible Patients with MIBC in Combination with Enfortumab Vedotin
The efficacy of intravenous pembrolizumab in combination with enfortumab vedotin as neoadjuvant treatment and continued after radical cystectomy as adjuvant treatment was investigated in KEYNOTE-905 (NCT03924895), an open-label, randomized, multicenter, active-controlled trial in patients with previously untreated MIBC with predominant urothelial carcinoma histology and who were candidates for radical cystectomy (RC) with pelvic lymph node dissection (PLND) but were ineligible for or declined cisplatin-based chemotherapy. The study excluded patients with primary non-bladder urothelial cancer (i.e., cancer of the ureter, urethra, or renal pelvis) and those with active autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression.
Randomization was stratified by tumor stage (T2N0 vs T3/T4aN0 vs T1-T4aN1), cisplatin-eligibility (cisplatin-ineligible vs cisplatin-eligible but declined), and geographic region (United States vs European Union vs Rest of World).
Patients were randomized 1:1 to receive:
- Neoadjuvant intravenous pembrolizumab 200 mg over 30 minutes as an intravenous infusion on Day 1 and enfortumab vedotin 1.25 mg/kg as an intravenous infusion on Days 1 and 8 of each 21-day cycle for 3 cycles prior to surgery, followed by adjuvant intravenous pembrolizumab 200 mg over 30 minutes on Day 1 of each 21-day cycle for 14 cycles and adjuvant enfortumab vedotin 1.25 mg/kg on Days 1 and 8 of each 21-day cycle for 6 cycles (n=170).
- Immediate RC and PLND alone (n=174).
Treatment continued until completion of study medications, disease progression, not undergoing or refusal of RC and PLND, disease recurrence in the adjuvant phase, or unacceptable toxicity. Assessment of tumor status, including CT/MRI, was performed at baseline, within 5 weeks prior to RC and PLND, and at 6 weeks post-radical cystectomy. Following RC and PLND, assessment of tumor status, including cystoscopy and urine cytology for patients who did not undergo surgery, was performed every 12 weeks up to 2 years, and every 24 weeks thereafter.
The study population characteristics were: median age of 73 years (range: 46 to 87 years); 78% male; 78% White, 16% Asian, 3.2% Multiple, 1.2% Black or African American, 0.3% American Indian or Alaska Native, and 1.2% missing; 6% Hispanic or Latino, 91% not Hispanic or Latino, and 2.9% not reported; 57% had ECOG PS of 0, 29% had ECOG PS of 1, and 14% had ECOG PS of 2; 18% were T2N0, 77% T3/T4aN0, and 4.9% T1-T4aN1. Ninety-one percent of patients had pure urothelial carcinoma histology; 4.4% had urothelial carcinoma with squamous differentiation, 2.6% had urothelial carcinoma with glandular differentiation, and 2% had urothelial carcinoma with other variant histology. Among the 281 patients who were ineligible for cisplatin, 72% had baseline creatinine clearance of 30-59 mL/min, 17% had ECOG PS of 2, 21% had Grade 2 or greater hearing loss, 3.9% had New York Heart Association Class III heart failure, and 13% met more than one cisplatin-ineligibility criterion.
A total of 149 (88%) patients in the intravenous pembrolizumab in combination with enfortumab vedotin arm and 156 (90%) patients in the RC and PLND alone arm underwent RC and PLND.
The trial was not designed to isolate the effect of intravenous pembrolizumab in each phase (neoadjuvant or adjuvant) of treatment.
The major efficacy outcome measure was EFS as assessed by BICR. Overall survival (OS) and pathological complete response (pCR) rate as assessed by blinded independent pathology review were additional efficacy outcome measures.
The trial demonstrated a statistically significant improvement in EFS and OS in patients treated with neoadjuvant and adjuvant intravenous pembrolizumab in combination with enfortumab vedotin compared with RC and PLND alone. Table 77 and Figures 19 and 20 summarize key efficacy measures.
Table 77: Efficacy Results for Perioperative Intravenous Pembrolizumab with Enfortumab Vedotin in KEYNOTE-905 Endpoint Intravenous
Pembrolizumab
200 mg every 3 weeks
in combination with
enfortumab vedotin before
and after RC with PLND
n=170RC with PLND alone
n=174NR = not reached
EFS is defined as time from randomization to the first of: disease progression preventing curative surgery, failure to undergo surgery for participants with muscle invasive residual disease, incomplete surgical resection, local or distant recurrence after surgery, or death.- * Based on Kaplan-Meier estimates
- † Based on stratified Cox regression model with Efron’s method of tie handling with treatment as a covariate
- ‡ Based on stratified log-rank test
EFS Number of patients with event (%) 48 (28%) 95 (55%) Median in months* (95% CI) NR (37.3, NR) 15.7 (10.3, 20.5) Hazard ratio† (95% CI) 0.40 (0.28, 0.57) p-Value‡ <0.0001 OS Number of patients with event (%) 38 (22%) 68 (39%) Median in months* (95% CI) NR (NR, NR) 41.7 (31.8, NR) Hazard ratio† (95% CI) 0.50 (0.33, 0.74) p-Value‡ 0.0002 Figure 19: Kaplan-Meier Curve for Event-Free Survival by Treatment Arm in KEYNOTE-905 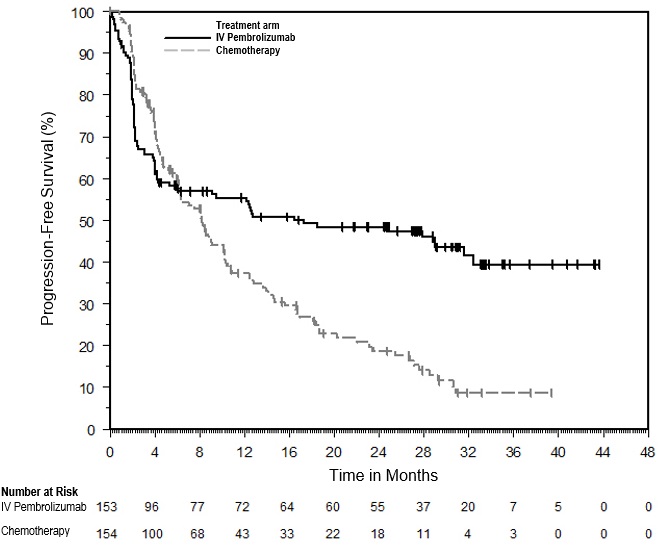
Figure 20: Kaplan-Meier Curve for Overall Survival by Treatment Arm in KEYNOTE-905 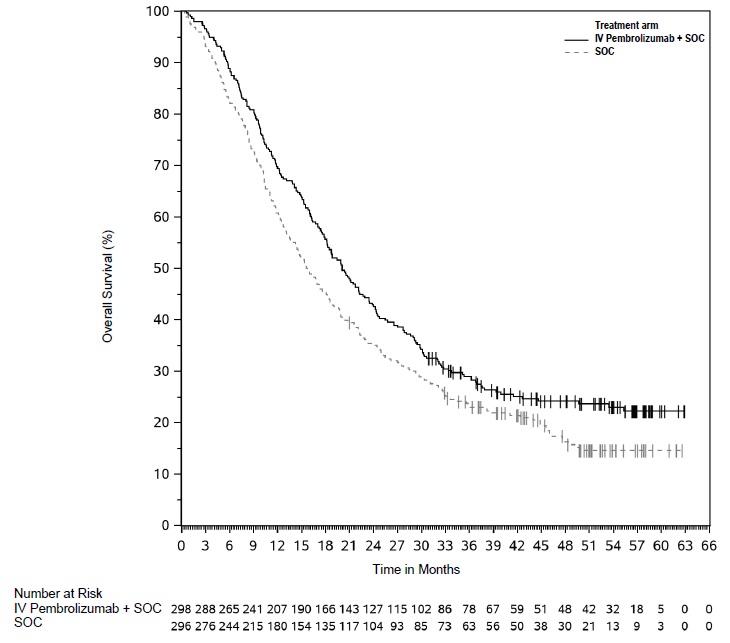
The trial demonstrated a statistically significant difference in pCR rate (57.1% [95% CI: 49.3, 64.6] vs. 8.6% [95% CI: 4.9, 13.8]; p<0.0001).
BCG-unresponsive High-Risk Non-Muscle Invasive Bladder Cancer
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-057 (NCT02625961), a multicenter, open-label, single-arm trial in 96 patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. BCG-unresponsive high-risk NMIBC was defined as persistent disease despite adequate BCG therapy, disease recurrence after an initial tumor-free state following adequate BCG therapy, or T1 disease following a single induction course of BCG. Adequate BCG therapy was defined as administration of at least five of six doses of an initial induction course plus either of: at least two of three doses of maintenance therapy or at least two of six doses of a second induction course. Prior to treatment, all patients had undergone transurethral resection of bladder tumor (TURBT) to remove all resectable disease (Ta and T1 components). Residual CIS (Tis components) not amenable to complete resection was allowed. The trial excluded patients with muscle invasive (i.e., T2, T3, T4) locally advanced non-resectable or metastatic urothelial carcinoma, concurrent extra-vesical (i.e., urethra, ureter or renal pelvis) non-muscle invasive transitional cell carcinoma of the urothelium, or autoimmune disease or a medical condition that required immunosuppression.
Patients received intravenous pembrolizumab 200 mg every 3 weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC, or progressive disease. Assessment of tumor status was performed every 12 weeks for two years and then every 24 weeks for three years, and patients without disease progression could be treated for up to 24 months. The major efficacy outcome measures were complete response (as defined by negative results for cystoscopy [with TURBT/biopsies as applicable], urine cytology, and computed tomography urography [CTU] imaging) and duration of response.
The study population characteristics were: median age of 73 years (range: 44 to 92); 44% age ≥75; 84% male; 67% White; and 73% and 27% with an ECOG performance status of 0 or 1, respectively. Tumor pattern at study entry was CIS with T1 (13%), CIS with high grade TA (25%), and CIS (63%). Baseline high-risk NMIBC disease status was 27% persistent and 73% recurrent. The median number of prior instillations of BCG was 12.
The median follow-up time was 28.0 months (range: 4.6 to 40.5 months). Efficacy results are summarized in Table 78.
Table 78: Efficacy Results in KEYNOTE-057 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=96+ Denotes ongoing response - * Based on patients (n=39) that achieved a complete response; reflects period from the time complete response was achieved
Complete Response Rate (95% CI) 41% (31, 51) Duration of Response* Median in months (range) 16.2 (0.0+, 30.4+) % (n) with duration ≥12 months 46% (18) 14.7 Microsatellite Instability-High or Mismatch Repair Deficient Cancer
The efficacy of intravenous pembrolizumab was investigated in 504 patients with MSI-H or dMMR cancers enrolled in three multicenter, non-randomized, open-label, multi-cohort trials: KEYNOTE-164 (NCT02460198), KEYNOTE-158 (NCT02628067), and KEYNOTE-051 (NCT02332668). All trials excluded patients with autoimmune disease or a medical condition that required immunosuppression. Regardless of histology, MSI or MMR tumor status was determined using polymerase chain reaction (PCR; local or central) or immunohistochemistry (IHC; local or central), respectively.
- KEYNOTE-164 enrolled 124 patients with advanced MSI-H or dMMR colorectal cancer (CRC) that progressed following treatment with fluoropyrimidine and either oxaliplatin or irinotecan +/- anti-VEGF/EGFR mAb-based therapy.
- KEYNOTE-158 enrolled 373 patients with advanced MSI-H or dMMR non-colorectal cancers (non-CRC) who had disease progression following prior therapy. Patients were either prospectively enrolled with MSI-H/dMMR tumors (Cohort K) or retrospectively identified in one of 10 solid tumor cohorts (Cohorts A-J).
- KEYNOTE-051 enrolled 7 pediatric patients with MSI-H or dMMR cancers.
Adult patients received intravenous pembrolizumab 200 mg every 3 weeks (pediatric patients received 2 mg/kg every 3 weeks) until unacceptable toxicity, disease progression, or a maximum of 24 months. In KEYNOTE-164 and KEYNOTE-158, assessment of tumor status was performed every 9 weeks through the first year, then every 12 weeks thereafter. In KEYNOTE-051, assessment of tumor status was performed every 8 weeks for 24 weeks, and then every 12 weeks thereafter. The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ in KEYNOTE-158) and as assessed by the investigator according to RECIST v1.1 in KEYNOTE-051.
In KEYNOTE-164 and KEYNOTE-158, the study population characteristics were median age of 60 years, 36% age 65 or older; 44% male; 78% White, 14% Asian, 4% American Indian or Alaska Native, and 3% Black; and 45% ECOG PS of 0 and 55% ECOG PS of 1. Ninety-two percent of patients had metastatic disease and 4% had locally advanced, unresectable disease. Thirty-seven percent of patients received one prior line of therapy and 61% received two or more prior lines of therapy.
In KEYNOTE-051, the study population characteristics were median age of 11 years (range: 3 to 16); 71% female; 86% White and 14% Asian; and 57% had a Lansky/Karnofsky Score of 100. Seventy-one percent of patients had Stage IV and 14% had Stage III disease. Fifty-seven percent of patients received one prior line of therapy and 29% received two prior lines of therapy.
Discordant results were observed between local MSI-H or dMMR tests and central testing among patients enrolled in Cohort K of KEYNOTE-158. Among 104 tumor samples that were MSI-H or dMMR by local testing and also tested using the FoundationOne®CDx (F1CDx) test, 59 (56.7%) were MSI-H and 45 (43.3%) were not MSI-H. Among 169 tumor samples that were MSI-H or dMMR by local testing and also tested using the VENTANA MMR RxDx Panel, 105 (62.1%) were dMMR and 64 (37.9%) were pMMR.
Efficacy results are summarized in Tables 79 and 80.
Table 79: Efficacy Results for Patients with MSI-H/dMMR Cancer Endpoint Intravenous Pembrolizumab
n=504*+ Denotes ongoing response - * Median follow-up time of 20.1 months (range 0.1 to 71.4 months)
- † Of the 7 pediatric patients from KEYNOTE-051, 1 patient had a radiographic complete response after initial growth of their tumor but is not reflected in the results.
Objective Response Rate ORR (95% CI)† 33.3% (29.2, 37.6) Complete response rate 10.3% Partial response rate 23.0% Duration of Response n=168 Median in months (range) 63.2 (1.9+, 63.9+) % with duration ≥12 months 77% % with duration ≥36 months 39% Table 80: Response by Tumor Type Objective Response Rate Duration of
Response rangeN n (%) 95% CI (months) + Denotes ongoing response - * Results include patients in Cohort K of KEYNOTE-158 that were later determined to be pMMR or not MSI-H by central testing
- † Includes 6 pediatric patients with brain cancer
- ‡ In addition to the 1 adult responder, 1 pediatric patient had a radiographic complete response after initial growth of their tumor.
- § Includes tumor type (n): anal (3), HNSCC (1), nasopharyngeal (1), retroperitoneal (1), testicular (1), vaginal (1), vulvar (1), appendiceal adenocarcinoma, NOS (1), hepatocellular carcinoma (1), and carcinoma of unknown origin (1). Includes 1 pediatric patient with abdominal adenocarcinoma.
CRC 124 42 (34%) (26%, 43%) (4.4, 58.5+) Non-CRC* 380 126 (33%) (28%, 38%) (1.9+, 63.9+) Endometrial cancer 94 47 (50%) (40%, 61%) (2.9, 63.2) Gastric or GE junction cancer 51 20 (39%) (26%, 54%) (1.9+, 63.0+) Small intestinal cancer 27 16 (59%) (39%, 78%) (3.7+, 57.3+) Brain cancer 27† 1 (4%)‡ (0%, 19%) 18.9 Ovarian cancer 25 8 (32%) (15%, 54%) (4.2, 56.6+) Biliary cancer 22 9 (41%) (21%, 64%) (6.2, 49.0+) Pancreatic cancer 22 4 (18%) (5%, 40%) (8.1, 24.3+) Sarcoma 14 3 (21%) (5%, 51%) (35.4+, 57.2+) Breast cancer 13 1 (8%) (0%,36%) 24.3+ Other§ 13 4 (31%) (9%, 61%) (6.2+, 32.3+) Cervical cancer 11 1 (9%) (0%, 41%) 63.9+ Neuroendocrine cancer 11 1 (9%) (0%, 41%) 13.3 Prostate cancer 8 1 (13%) (0%, 53%) 24.5+ Adrenocortical cancer 7 1 (14%) (0%, 58%) 4.2 Mesothelioma 7 0 (0%) (0%, 41%) Thyroid cancer 7 1 (14%) (0%, 58%) 8.2 Small cell lung cancer 6 2 (33%) (4%, 78%) (20.0, 47.5) Bladder cancer 6 3 (50%) (12%, 88%) (35.6+, 57.5+) Salivary cancer 5 2 (40%) (5%, 85%) (42.6+, 57.8+) Renal cell cancer 4 1 (25%) (0%, 81%) 22.0 Exploratory analysis by TMB
In an exploratory analysis performed in 138 patients (Cohort K of KEYNOTE-158) who were tested retrospectively for tumor mutation burden (TMB) using an FDA-authorized test, 45 (33%) had tumors with TMB score of <10 mut/Mb; ORR in these 45 patients was 6.7% (95% CI: 1.4, 18.3). Among the 45 patients with TMB score of <10 mut/Mb, 39 of the patients were pMMR/not MSI-H when tested using an FDA-authorized test.
14.8 Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-177 (NCT02563002), a multicenter, randomized, open-label, active-controlled trial that enrolled 307 patients with previously untreated unresectable or metastatic MSI-H or dMMR CRC. MSI or MMR tumor status was determined locally using polymerase chain reaction (PCR) or immunohistochemistry (IHC), respectively. Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible.
Patients were randomized (1:1) to receive intravenous pembrolizumab 200 mg intravenously every 3 weeks or investigator’s choice of the following chemotherapy regimens given intravenously every 2 weeks:
- mFOLFOX6 (oxaliplatin, leucovorin, and FU) or mFOLFOX6 in combination with either bevacizumab or cetuximab: Oxaliplatin 85 mg/m2, leucovorin 400 mg/m2 (or levoleucovorin 200 mg/m2), and FU 400 mg/m2 bolus on Day 1, then FU 2400 mg/m2 over 46-48 hours. Bevacizumab 5 mg/kg on Day 1 or cetuximab 400 mg/m2 on first infusion, then 250 mg/m2 weekly.
- FOLFIRI (irinotecan, leucovorin, and FU) or FOLFIRI in combination with either bevacizumab or cetuximab: Irinotecan 180 mg/m2, leucovorin 400 mg/m2 (or levoleucovorin 200 mg/m2), and FU 400 mg/m2 bolus on Day 1, then FU 2400 mg/m2 over 46-48 hours. Bevacizumab 5 mg/kg on Day 1 or cetuximab 400 mg/m2 on first infusion, then 250 mg/m2 weekly.
Treatment with intravenous pembrolizumab or chemotherapy continued until RECIST v1.1-defined progression of disease as determined by the investigator or unacceptable toxicity. Patients treated with intravenous pembrolizumab without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks. Patients randomized to chemotherapy were offered intravenous pembrolizumab at the time of disease progression. The main efficacy outcome measures were PFS (as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ) and OS. Additional efficacy outcome measures were ORR and DoR.
A total of 307 patients were enrolled and randomized to intravenous pembrolizumab (n=153) or chemotherapy (n=154). The baseline characteristics of these 307 patients were: median age of 63 years (range: 24 to 93), 47% age 65 or older; 50% male; 75% White and 16% Asian; 52% had an ECOG PS of 0 and 48% had an ECOG PS of 1; and 27% received prior adjuvant or neoadjuvant chemotherapy. Among 154 patients randomized to receive chemotherapy,143 received chemotherapy per the protocol. Of the 143 patients, 56% received mFOLFOX6, 44% received FOLFIRI, 70% received bevacizumab plus mFOLFOX6 or FOLFIRI, and 11% received cetuximab plus mFOLFOX6 or FOLFIRI.
The trial demonstrated a statistically significant improvement in PFS for patients randomized to intravenous pembrolizumab compared with chemotherapy. There was no statistically significant difference between intravenous pembrolizumab and chemotherapy in the final OS analysis. Sixty percent of the patients who had been randomized to receive chemotherapy had crossed over to receive subsequent anti-PD-1/PD-L1 therapies including intravenous pembrolizumab. The median follow-up time at the final analysis was 38.1 months (range: 0.2 to 58.7 months). Table 81 and Figure 21 summarize the key efficacy measures for KEYNOTE-177.
Table 81: Efficacy Results in Patients with MSI-H or dMMR CRC in KEYNOTE-177 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=153Chemotherapy
n=154+ Denotes ongoing response
NR = not reached- * Based on Cox regression model
- † Two-sided p-Value based on log-rank test (compared to a significance level of 0.0234)
- ‡ Final OS analysis
- § Two-sided p-Value based on log-rank test (compared to a significance level of 0.0492)
- ¶ Based on confirmed response by BICR review
- # Based on n=67 patients with a response in the intravenous pembrolizumab arm and n=51 patients with a response in the chemotherapy arm
- Þ Based on observed duration of response
PFS Number (%) of patients with event 82 (54%) 113 (73%) Median in months (95% CI) 16.5 (5.4, 32.4) 8.2 (6.1, 10.2) Hazard ratio* (95% CI) 0.60 (0.45, 0.80) p-Value† 0.0004 OS‡ Number (%) of patients with event 62 (41%) 78 (51%) Median in months (95% CI) NR (49.2, NR) 36.7 (27.6, NR) Hazard ratio* (95% CI) 0.74 (0.53, 1.03) p-Value§ 0.0718 Objective Response Rate¶ ORR (95% CI) 44% (35.8, 52.0) 33% (25.8, 41.1) Complete response rate 11% 4% Partial response rate 33% 29% Duration of Response¶,# Median in months (range) NR (2.3+, 41.4+) 10.6 (2.8, 37.5+) % with duration ≥12 monthsÞ 75% 37% % with duration ≥24 monthsÞ 43% 18% Figure 21: Kaplan-Meier Curve for PFS in KEYNOTE-177 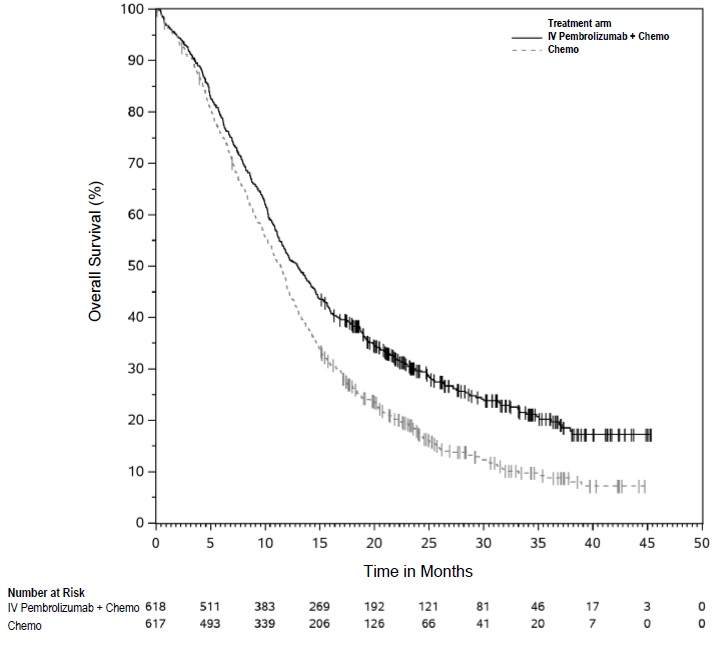
14.9 Gastric Cancer
First-line Treatment of Locally Advanced Unresectable or Metastatic HER2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma for Tumors Expressing PD-L1 (CPS ≥1)
The efficacy of intravenous pembrolizumab in combination with trastuzumab plus fluoropyrimidine and platinum chemotherapy was investigated in KEYNOTE-811 (NCT03615326), a multicenter, randomized, double-blind, placebo-controlled trial that enrolled 698 patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma who had not previously received systemic therapy for metastatic disease. PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx assay. Patients with an autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by PD-L1 expression (CPS ≥1 or CPS <1), chemotherapy regimen (5-FU plus cisplatin [FP] or capecitabine plus oxaliplatin [CAPOX]), and geographic region (Europe/Israel/North America/Australia, Asia, or Rest of the World). Patients were randomized (1:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg, trastuzumab 8 mg/kg on first infusion and 6 mg/kg in subsequent cycles, followed by investigator’s choice of combination chemotherapy of cisplatin 80 mg/m2 for up to 6 cycles and 5-FU 800 mg/m2/day for 5 days (FP) or oxaliplatin 130 mg/m2 up to 6-8 cycles and capecitabine 1000 mg/m2 bid for 14 days (CAPOX).
- Placebo, trastuzumab 8 mg/kg on first infusion and 6 mg/kg in subsequent cycles, followed by investigator’s choice of combination chemotherapy of cisplatin 80 mg/m2 for up to 6 cycles and 5-FU 800 mg/m2/day for 5 days (FP) or oxaliplatin 130 mg/m2 up to 6-8 cycles and capecitabine 1000 mg/m2 bid for 14 days (CAPOX).
All study medications, except oral capecitabine, were administered as an intravenous infusion every 3-week cycle. Treatment with intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. The major outcome measures assessed were PFS by BICR using RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and OS.
Additional outcome measures included ORR and DoR, based on BICR using RECIST 1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
Among the 698 patients randomized, 594 (85%) had tumors that expressed PD-L1 with a CPS ≥1. PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx assay. The population characteristics of these 594 patients were: median age of 63 years (range: 19 to 85), 43% age 65 or older; 80% male; 63% White, 33% Asian, and 0.7% Black; 42% ECOG PS of 0 and 58% ECOG PS of 1. Ninety-eight percent of patients had metastatic disease (Stage IV) and 2% had locally advanced unresectable disease. Ninety-five percent (n=562) had tumors that were not MSI-H, 1% (n=8) had tumors that were MSI-H, and in 4% (n=24) the status was not known. Eighty-five percent of patients received CAPOX.
A statistically significant improvement in OS and PFS was demonstrated in patients randomized to intravenous pembrolizumab in combination with trastuzumab and chemotherapy compared with placebo in combination with trastuzumab and chemotherapy; however, an exploratory analysis of OS in the PD-L1 CPS <1 population showed a HR of 1.10 (95% CI: 0.72, 1.68), indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 CPS ≥1.
Efficacy results at the final analysis for the subgroup of patients whose tumors expressed PD-L1 with a CPS ≥1 are summarized in Table 82 and Figure 22.
Table 82: Efficacy Results for KEYNOTE-811 with PD-L1 Expression CPS ≥1 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
Trastuzumab
Fluoropyrimidine and Platinum Chemotherapy
n=298Placebo
Trastuzumab
Fluoropyrimidine and
Platinum Chemotherapy
n=296+ Denotes ongoing response - * Based on Kaplan-Meier estimation
- † Based on the unstratified Cox proportional hazard model
- ‡ Response: Best objective response as confirmed complete response or partial response
OS Number (%) of patients with event 226 (76%) 244 (82%) Median in months* (95% CI) 20.1 (17.9, 22.9) 15.7 (13.5, 18.5) Hazard ratio† (95% CI) 0.79 (0.66, 0.95) PFS Number (%) of patients with event 221 (74%) 226 (76%) Median in months* (95% CI) 10.9 (8.5, 12.5) 7.3 (6.8, 8.4) Hazard ratio† (95% CI) 0.72 (0.60, 0.87) Objective Response Rate ORR‡ (95% CI) 73% (68, 78) 58% (53, 64) Complete response rate 17% 10% Partial response rate 56% 48% Duration of Response n=218 n=173 Median in months* (95% CI)
Range in months11.3 (9.9, 13.7)
1.1+, 60.8+9.6 (7.1, 11.2)
1.4+, 60.5+Figure 22: Kaplan-Meier Curve for Overall Survival by Treatment Arm in KEYNOTE-811 (CPS ≥1) 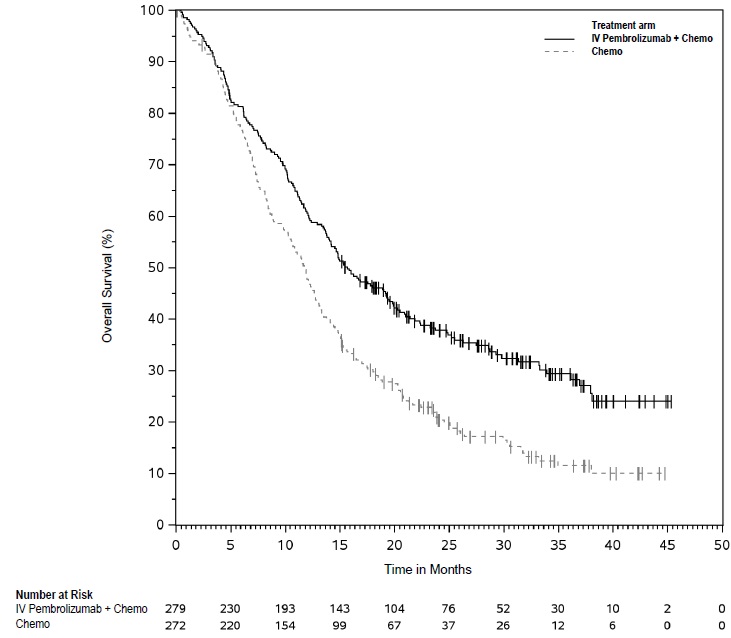
First-line Treatment of Locally Unresectable or Metastatic HER2-Negative Gastric or Gastroesophageal Junction Adenocarcinoma for Tumors Expressing PD-L1 (CPS ≥1)
The efficacy of intravenous pembrolizumab in combination with fluoropyrimidine- and platinum-containing chemotherapy was investigated in KEYNOTE-859 (NCT03675737), a multicenter, randomized, double-blind, placebo-controlled trial that enrolled 1579 patients with HER2-negative advanced gastric or GEJ adenocarcinoma who had not previously received systemic therapy for metastatic disease. Patients with an autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by PD-L1 expression (CPS ≥1 or CPS <1), chemotherapy regimen (FP or CAPOX), and geographic region (Europe/Israel/North America/Australia, Asia, or Rest of the World). Patients were randomized (1:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg, investigator’s choice of combination chemotherapy of cisplatin 80 mg/m2 and 5-FU 800 mg/m2/day for 5 days (FP) or oxaliplatin 130 mg/m2 and capecitabine 1000 mg/m2 bid for 14 days (CAPOX).
- Placebo, investigator’s choice of combination chemotherapy of cisplatin 80 mg/m2 and 5-FU 800 mg/m2/day for 5 days (FP) or oxaliplatin 130 mg/m2 and capecitabine 1000 mg/m2 bid for 14 days (CAPOX).
All study medications, except oral capecitabine, were administered as an intravenous infusion every 3-week cycle. Platinum agents could be administered for 6 or more cycles following local guidelines. Treatment with intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. The major efficacy outcome measure was OS. Additional secondary efficacy outcome measures included PFS, ORR, and DoR as assessed by BICR using RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
Among 1,579 patients, 1,235 (78%) had tumors expressing PD-L1 CPS ≥ 1. The population characteristics in patients with PD-L1 CPS ≥ 1 expressing tumors were: median age of 62 years (range: 24 to 86), 40% age 65 or older; 70% male and 30% female; 55% White, 33% Asian, 4.6% Multiple, 4.3% American Indian or Alaskan Native, 1.3% Black, and 0.2% Native Hawaiian or other Pacific Islander; 76% Not Hispanic or Latino and 21% Hispanic or Latino; 37% ECOG PS of 0 and 63% ECOG PS of 1. Ninety-six percent of patients had metastatic disease (Stage IV) and 3% had locally advanced unresectable disease. Five percent (n=66) had tumors that were MSI-H. Eighty-six percent of patients received CAPOX.
A statistically significant improvement in OS, PFS, and ORR was demonstrated in patients randomized to intravenous pembrolizumab in combination with chemotherapy compared with placebo in combination with chemotherapy at the time of a pre-specified interim analysis of OS; however, an exploratory analysis of OS in the PD-L1 CPS <1 population showed a HR of 0.92 (95% CI 0.73, 1.17) indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 CPS ≥1. Efficacy results for patients whose tumors expressed PD-L1 CPS ≥1 and CPS ≥10 are summarized in Table 83 and Figures 23 and 24.
Table 83: Efficacy Results* for KEYNOTE-859 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
and
FP or CAPOX
n=618Placebo
and
FP or CAPOX
n=617Intravenous Pembrolizumab
200 mg every 3 weeks
and
FP or CAPOX
n=279Placebo
and
FP or CAPOX
n=272CPS ≥1 CPS ≥10 + Denotes ongoing response - * Based on a pre-specified interim analysis
- † Based on the stratified Cox proportional hazard model
- ‡ One-sided p-Value based on stratified log-rank test
- § Response: Best objective response as confirmed complete response or partial response
- ¶ One-sided p-Value based on stratified Miettinen & Nurminen method
- # Based on Kaplan-Meier estimates
OS Number (%) of patients with event 464 (75) 526 (85) 188 (67) 226 (83) Median in months (95% CI) 13.0 (11.6, 14.2) 11.4 (10.5, 12.0) 15.7 (13.8, 19.3) 11.8 (10.3, 12.7) Hazard ratio† (95% CI) 0.74 (0.65, 0.84) 0.65 (0.53, 0.79) p-Value (stratified log-rank)‡ <0.0001 <0.0001 PFS Number (%) of patients with event 443 (72%) 483 (78%) 190 (68) 210 (77) Median in months (95% CI) 6.9 (6.0, 7.2) 5.6 (5.4, 5.7) 8.1 (6.8, 8.5) 5.6 (5.4, 6.7) Hazard ratio† (95% CI) 0.72 (0.63, 0.82) 0.62 (0.51, 0.76) p-Value (stratified log-rank)‡ <0.0001 <0.0001 Objective Response Rate ORR§ (95% CI) 52% (48, 56) 43% (39, 47) 61% (55, 66) 43% (37, 49) Complete response rate 10% 6% 13% 5% Partial response rate 42% 37% 48% 38% p-Value¶ 0.0004 <0.0001 Duration of Response n=322 n=263 n=169 n=117 Median in months# (95% CI) 8.3 (7.0, 10.9) 5.6 (5.4, 6.9) 10.9 (8.0, 13.8) 5.8 (5.3, 7.0) Range in months 1.2+, 41.5+ 1.3+, 34.2+ 1.2+, 41.5+ 1.4+, 31.2+ Figure 23: Kaplan-Meier Curve for Overall Survival in KEYNOTE-859 (CPS ≥1) 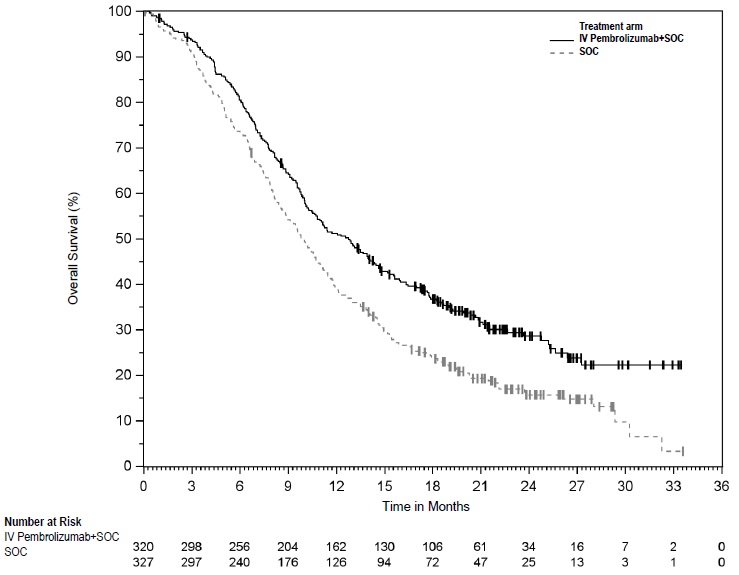
Figure 24: Kaplan-Meier Curve for Overall Survival in KEYNOTE-859 (CPS ≥10) 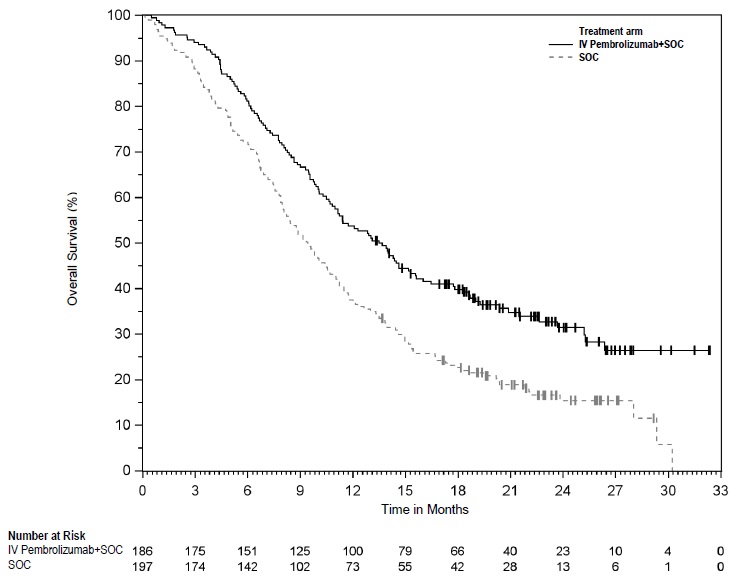
An exploratory analysis of OS in the 74 patients with MSI-H tumors irrespective of PD-L1 status showed a HR of 0.34 (95% CI: 0.18, 0.66).
14.10 Esophageal Cancer
First-line Treatment of Locally Advanced Unresectable or Metastatic Esophageal/Gastroesophageal Junction Cancer for Tumors Expressing PD-L1 (CPS ≥1)
KEYNOTE-590
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-590 (NCT03189719), a multicenter, randomized, placebo-controlled trial that enrolled 749 patients with metastatic or locally advanced esophageal or gastroesophageal junction (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma who were not candidates for surgical resection or definitive chemoradiation. PD-L1 status was centrally determined in tumor specimens in all patients using the PD-L1 IHC 22C3 pharmDx assay. Patients with active autoimmune disease, a medical condition that required immunosuppression, or who received prior systemic therapy in the locally advanced or metastatic setting were ineligible. Randomization was stratified by tumor histology (squamous cell carcinoma vs. adenocarcinoma), geographic region (Asia vs. ex-Asia), and ECOG performance status (0 vs. 1).
Patients were randomized (1:1) to one of the following treatment arms; all study medications were administered via intravenous infusion:
- Intravenous pembrolizumab 200 mg on Day 1 of each three-week cycle in combination with cisplatin 80 mg/m2 IV on Day 1 of each three-week cycle for up to six cycles and FU 800 mg/m2 IV per day on Day 1 to Day 5 of each three-week cycle, or per local standard for FU administration, for up to 24 months.
- Placebo on Day 1 of each three-week cycle in combination with cisplatin 80 mg/m2 IV on Day 1 of each three-week cycle for up to six cycles and FU 800 mg/m2 IV per day on Day 1 to Day 5 of each three-week cycle, or per local standard for FU administration, for up to 24 months.
Treatment with intravenous pembrolizumab or chemotherapy continued until unacceptable toxicity or disease progression. Patients could be treated with intravenous pembrolizumab for up to 24 months in the absence of disease progression. The major efficacy outcome measures were OS and PFS as assessed by the investigator according to RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ). The study pre-specified analyses of OS and PFS based on squamous cell histology, CPS ≥10, and in all patients. Additional efficacy outcome measures were ORR and DoR, according to modified RECIST v1.1, as assessed by the investigator. Additional analyses of efficacy outcome measures were also conducted based on PD-L1 CPS ≥1.
Among 749 patients, 647 (86%) had tumors expressing PD-L1 CPS ≥ 1. The study population characteristics in patients with PD-L1 CPS ≥ 1 expressing tumors were: median age of 63 years (range: 27 to 89), 41% age 65 or older; 83% male; 36% White, 54% Asian, and 1% Black; 40% had an ECOG PS of 0 and 59% had an ECOG PS of 1. Ninety-one percent had M1 disease and 9% had M0 disease. Seventy-four percent had a tumor histology of squamous cell carcinoma, and 26% had adenocarcinoma.
The trial demonstrated a statistically significant improvement in OS and PFS for patients randomized to intravenous pembrolizumab in combination with chemotherapy compared to chemotherapy; however, an exploratory analysis of OS in the PD-L1 CPS <1 population showed an HR of 0.96 (0.59, 1.55), indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 CPS ≥1.
Table 84 and Figures 25 and 26 summarize the efficacy results for KEYNOTE-590 in patients whose tumors expressed PD-L1 CPS ≥1 and CPS ≥10.
Table 84: Efficacy Results in Patients with Locally Advanced Unresectable or Metastatic Esophageal Cancer in KEYNOTE-590 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
Cisplatin
FU
n=320Placebo
Cisplatin
FU
n=327Intravenous Pembrolizumab
200 mg every 3 weeks
Cisplatin
FU
n=186Placebo
Cisplatin
FU
n=197CPS ≥1 CPS ≥10 - * Based on the stratified Cox proportional hazard model
- † Based on a stratified log-rank test; p-Value for CPS ≥1 not included (not pre-specified subgroup)
- ‡ Confirmed complete response or partial response
OS Number (%) of events 222 (69) 271 (83) 124 (67) 165 (84) Median in months
(95% CI)12.7
(10.5, 14.4)9.8
(8.8, 10.8)13.5
(11.1, 15.6)9.4
(8.0, 10.7)Hazard ratio* (95% CI) 0.71 (0.59, 0.84) 0.62 (0.49, 0.78) p-Value† <0.0001 PFS Number of events (%) 252 (79) 291 (89) 140 (75) 174 (88) Median in months
(95% CI)6.3
(6.2, 7.1)5.7
(4.6, 6.0)7.5
(6.2, 8.2)5.5
(4.3, 6.0)Hazard ratio* (95% CI) 0.62 (0.52, 0.73) 0.51 (0.41, 0.65) p-Value† <0.0001 Objective Response Rate ORR, %‡
(95% CI)45
(40, 51)29
(24, 34)51
(44, 59)27
(21, 34)Number (%) of complete responses 19 (6) 9 (2.8) 11 (6) 5 (2.5) Number (%) of partial responses 126 (39) 85 (26) 84 (45) 48 (24) Duration of Response Median in months
(range)8.6
(1.2+, 31.0+)5.8
(1.5+, 25.0+)10.4
(1.9+, 28.9+)5.6
(1.5+, 25.0+)Figure 25: Kaplan-Meier Curve for Overall Survival in KEYNOTE-590 (CPS ≥1) 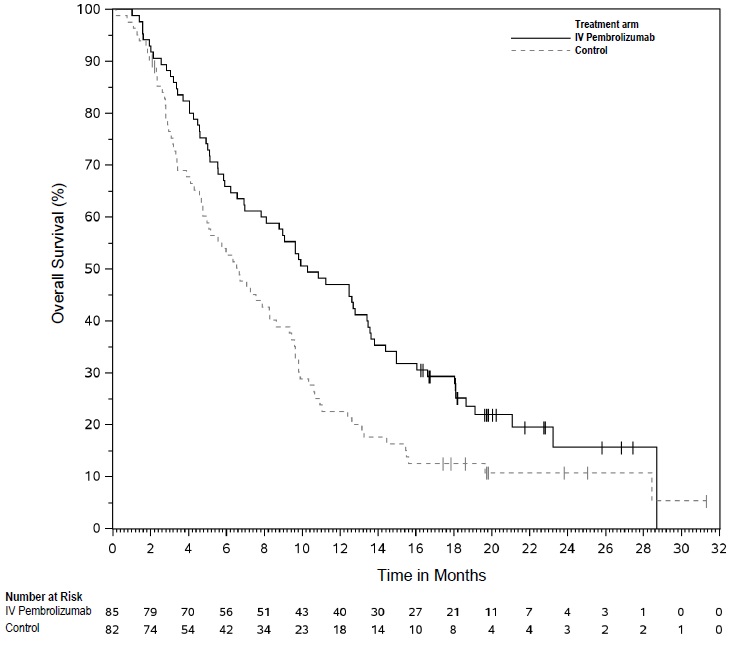
Figure 26: Kaplan-Meier Curve for Overall Survival in KEYNOTE-590 (CPS ≥10) 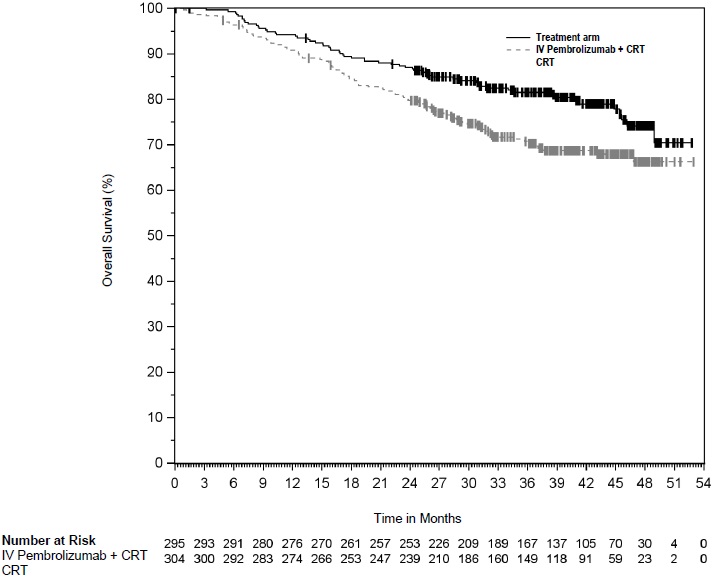
In a pre-specified formal test of OS in patients with PD-L1 CPS ≥10 (n=383), the median was 13.5 months (95% CI: 11.1, 15.6) for the intravenous pembrolizumab arm and 9.4 months (95% CI: 8.0, 10.7) for the placebo arm, with a HR of 0.62 (95% CI: 0.49, 0.78; p-Value < 0.0001). In an exploratory analysis, in patients with PD-L1 CPS <10 (n=347), the median OS was 10.5 months (95% CI: 9.7, 13.5) for the intravenous pembrolizumab arm and 10.6 months (95% CI: 8.8, 12.0) for the placebo arm, with a HR of 0.86 (95% CI: 0.68, 1.10).
Previously Treated Recurrent Locally Advanced or Metastatic Esophageal Cancer for Tumors Expressing PD-L1 (CPS≥ 10)
KEYNOTE-181
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-181 (NCT02564263), a multicenter, randomized, open-label, active-controlled trial that enrolled 628 patients with recurrent locally advanced or metastatic esophageal cancer who progressed on or after one prior line of systemic treatment for advanced disease. Patients with HER2/neu positive esophageal cancer were required to have received treatment with approved HER2/neu targeted therapy. All patients were required to have tumor specimens for PD-L1 testing at a central laboratory; PD-L1 status was determined using the PD-L1 IHC 22C3 pharmDx assay. Patients with a history of non-infectious pneumonitis that required steroids or current pneumonitis, active autoimmune disease, or a medical condition that required immunosuppression were ineligible.
Patients were randomized (1:1) to receive either intravenous pembrolizumab 200 mg every 3 weeks or investigator's choice of any of the following chemotherapy regimens, all given intravenously: paclitaxel 80-100 mg/m2 on Days 1, 8, and 15 of every 4-week cycle, docetaxel 75 mg/m2 every 3 weeks, or irinotecan 180 mg/m2 every 2 weeks. Randomization was stratified by tumor histology (esophageal squamous cell carcinoma [ESCC] vs. esophageal adenocarcinoma [EAC]/Siewert type I EAC of the gastroesophageal junction [GEJ]), and geographic region (Asia vs. ex-Asia). Treatment with intravenous pembrolizumab or chemotherapy continued until unacceptable toxicity or disease progression. Patients randomized to intravenous pembrolizumab were permitted to continue beyond the first RECIST v1.1 (modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ)-defined disease progression if clinically stable until the first radiographic evidence of disease progression was confirmed at least 4 weeks later with repeat imaging. Patients treated with intravenous pembrolizumab without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks. The major efficacy outcome measure was OS evaluated in the following co-primary populations: patients with ESCC, patients with tumors expressing PD-L1 CPS ≥10, and all randomized patients. Additional efficacy outcome measures were PFS, ORR, and DoR, according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR.
A total of 628 patients were enrolled and randomized to intravenous pembrolizumab (n=314) or investigator's treatment of choice (n=314). Of these 628 patients, 167 (27%) had ESCC that expressed PD-L1 with a CPS ≥10. Of these 167 patients, 85 patients were randomized to intravenous pembrolizumab and 82 patients to investigator's treatment of choice [paclitaxel (n=50), docetaxel (n=19), or irinotecan (n=13)]. The baseline characteristics of these 167 patients were: median age of 65 years (range: 33 to 80), 51% age 65 or older; 84% male; 32% White and 68% Asian; 38% had an ECOG PS of 0 and 62% had an ECOG PS of 1. Ninety percent had M1 disease and 10% had M0 disease. Prior to enrollment, 99% of patients had received platinum-based treatment and 84% had also received treatment with a fluoropyrimidine. Thirty-three percent of patients received prior treatment with a taxane.
The observed OS hazard ratio was 0.77 (95% CI: 0.63, 0.96) in patients with ESCC, 0.70 (95% CI: 0.52, 0.94) in patients with tumors expressing PD-L1 CPS ≥10, and 0.89 (95% CI: 0.75, 1.05) in all randomized patients. On further examination in patients whose ESCC tumors expressed PD-L1 (CPS ≥10), an improvement in OS was observed among patients randomized to intravenous pembrolizumab as compared with chemotherapy. Table 85 and Figure 27 summarize the key efficacy measures for KEYNOTE-181 for patients with ESCC CPS ≥10.
Table 85: Efficacy Results in Patients with Recurrent or Metastatic ESCC (CPS ≥10) in KEYNOTE-181 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=85Chemotherapy
n=82- * Based on the Cox regression model stratified by geographic region (Asia vs. ex-Asia)
OS Number (%) of patients with event 68 (80%) 72 (88%) Median in months (95% CI) 10.3 (7.0, 13.5) 6.7 (4.8, 8.6) Hazard ratio* (95% CI) 0.64 (0.46, 0.90) PFS Number (%) of patients with event 76 (89%) 76 (93%) Median in months (95% CI) 3.2 (2.1, 4.4) 2.3 (2.1, 3.4) Hazard ratio* (95% CI) 0.66 (0.48, 0.92) Objective Response Rate ORR (95% CI) 22 (14, 33) 7 (3, 15) Number (%) of complete responses 4 (5) 1 (1) Number (%) of partial responses 15 (18) 5 (6) Median duration of response in months (range) 9.3 (2.1+, 18.8+) 7.7 (4.3, 16.8+) Figure 27: Kaplan-Meier Curve for Overall Survival in KEYNOTE-181 (ESCC CPS ≥10) 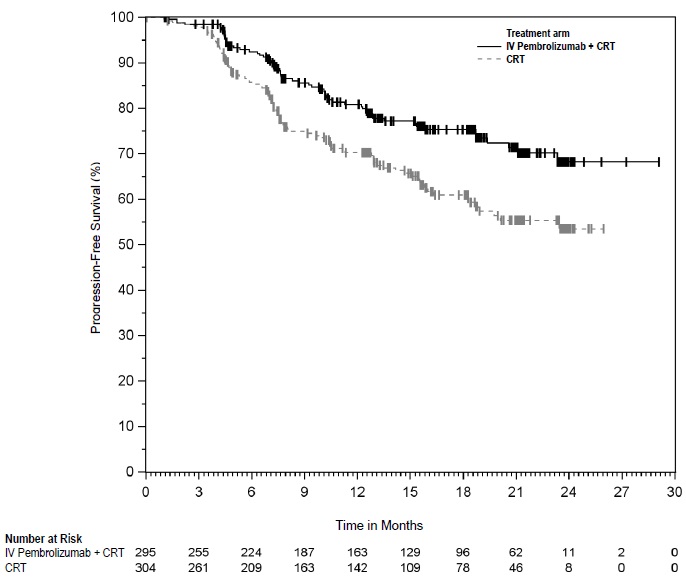
KEYNOTE-180
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-180 (NCT02559687), a multicenter, non-randomized, open-label trial that enrolled 121 patients with locally advanced or metastatic esophageal cancer who progressed on or after at least 2 prior systemic treatments for advanced disease. With the exception of the number of prior lines of treatment, the eligibility criteria were similar to and the dosage regimen identical to KEYNOTE-181.
The major efficacy outcome measures were ORR and DoR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR.
Among the 121 patients enrolled, 29% (n=35) had ESCC that expressed PD-L1 CPS ≥10. The baseline characteristics of these 35 patients were: median age of 65 years (range: 47 to 81), 51% age 65 or older; 71% male; 26% White and 69% Asian; 40% had an ECOG PS of 0 and 60% had an ECOG PS of 1. One hundred percent had M1 disease.
The ORR in the 35 patients with ESCC expressing PD-L1 was 20% (95% CI: 8, 37). Among the 7 responding patients, the DoR ranged from 4.2 to 25.1+ months, with 5 patients (71%) having responses of 6 months or longer and 3 patients (57%) having responses of 12 months or longer.
14.11 Cervical Cancer
FIGO 2014 Stage III-IVA Cervical Cancer with Chemoradiotherapy
The efficacy of intravenous pembrolizumab in combination with CRT (cisplatin and external beam radiation therapy [EBRT] followed by brachytherapy [BT]) was investigated in KEYNOTE-A18 (NCT04221945), a multicenter, randomized, double-blind, placebo-controlled trial that enrolled 1060 patients with cervical cancer who had not previously received any definitive surgery, radiation, or systemic therapy for cervical cancer. There were 599 patients with FIGO 2014 Stage III-IVA disease (tumor involves the lower third of the vagina or the pelvic sidewall, or there is hydronephrosis/non-functioning kidney or spread to adjacent pelvic organs, all without spread to distant organs), and 459 patients with FIGO 2014 Stage IB2-IIB disease (clinical lesion >4 cm confined to the cervix, or clinical lesion of any size with extension beyond the uterus, but which has not extended to the pelvic wall or lower third of the vagina) with positive nodes. Two patients had FIGO 2014 Stage IVB disease. Randomization was stratified by planned type of EBRT (Intensity-modulated radiation therapy [IMRT] or volumetric modulated arc therapy [VMAT] vs. non-IMRT and non-VMAT), stage at screening of cervical cancer (FIGO 2014 Stage IB2-IIB vs. FIGO 2014 Stage III-IVA), and planned total radiotherapy dose (EBRT + brachytherapy dose of <70 Gy vs. ≥70 Gy as per equivalent dose [EQD2]).
Patients were randomized (1:1) to one of two treatment arms:
- Intravenous pembrolizumab 200 mg IV every 3 weeks (5 cycles) concurrent with cisplatin 40 mg/m2 IV weekly (5 cycles, an optional sixth infusion could be administered per local practice), and radiotherapy (EBRT followed by BT), followed by intravenous pembrolizumab 400 mg IV every 6 weeks (15 cycles)
- Placebo IV every 3 weeks (5 cycles) concurrent with cisplatin 40 mg/m2 IV weekly (5 cycles, an optional sixth infusion could be administered per local practice), and radiotherapy (EBRT followed by BT), followed by placebo IV every 6 weeks (15 cycles)
Treatment continued until RECIST v1.1-defined progression of disease as determined by investigator or unacceptable toxicity.
Assessment of tumor status was performed every 12 weeks from completion of CRT for the first two years, followed by every 24 weeks in year 3, and then annually. The major efficacy outcome measures were PFS as assessed by investigator according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, or histopathologic confirmation, and OS.
Among the 599 patients with FIGO 2014 Stage III-IVA disease, the baseline characteristics were: median age of 52 years (range: 22 to 87), 17% age 65 or older; 36% White, 34% Asian, 2% Black; 38% Hispanic or Latino; 68% ECOG PS 0 and 32% ECOG PS 1; 93% with CPS ≥1; 71% had positive pelvic and/or positive para-aortic lymph node(s) and 29% had neither positive pelvic nor para-aortic lymph node(s); 83% had squamous cell carcinoma and 17% had non-squamous histology. Regarding radiation, 86% of patients received IMRT or VMAT EBRT, and the median EQD2 dose was 87 Gy (range: 7 to 114).
The trial demonstrated statistically significant improvements in PFS and OS in the ITT population. Exploratory analyses of PFS and OS by the stratification factor of FIGO 2014 stage showed that the improvement in the ITT population was primarily attributed to the results seen in the patients with FIGO 2014 Stage III-IVA disease. Table 86 and Figures 28 and 29 summarize the results in exploratory subgroup analyses of 599 patients with FIGO 2014 Stage III-IVA disease.
Table 86: Efficacy Results in KEYNOTE-A18 (Patients with FIGO 2014 Stage III-IVA Cervical Cancer) Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks and 400 mg every 6 weeks
with CRT
n=295Placebo
with CRT
n=304CRT = Chemoradiotherapy
NR = not reached- * Results at the time of pre-specified final analysis for OS
- † Based on the unstratified Cox proportional hazard model
- ‡ Results at the time of first pre-specified interim analysis for PFS
OS* Number of patients with event (%) 61 (21) 90 (30) Hazard ratio† (95% CI) 0.65 (0.47, 0.90) PFS by Investigator‡ Number of patients with event (%) 61 (21) 94 (31) Median in months (95% CI) NR (NR, NR) NR (18.8, NR) 12-month PFS rate (95% CI) 81% (75, 85) 70% (64, 76) Hazard ratio† (95% CI) 0.59 (0.43, 0.81) Figure 28: Kaplan-Meier Curve for Overall Survival in KEYNOTE-A18 (Patients with FIGO 2014 Stage III-IVA Cervical Cancer) 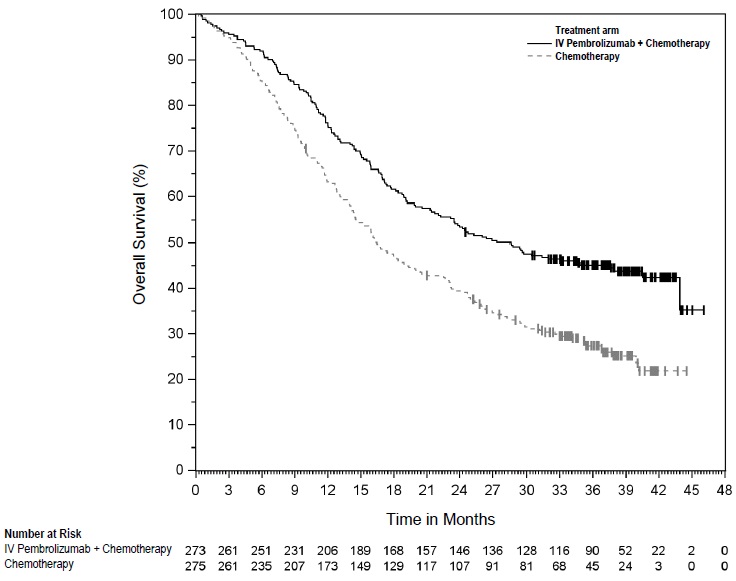
Figure 29: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-A18 (Patients with FIGO 2014 Stage III-IVA Cervical Cancer) 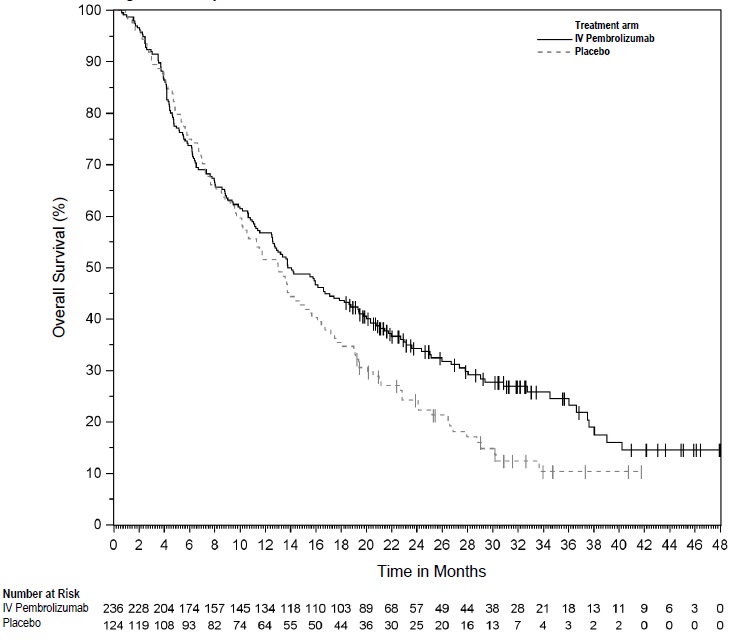
Persistent, Recurrent, or Metastatic Cervical Cancer for Tumors Expressing PD-L1 (CPS ≥1)
The efficacy of intravenous pembrolizumab in combination with paclitaxel and cisplatin or paclitaxel and carboplatin, with or without bevacizumab, was investigated in KEYNOTE-826 (NCT03635567), a multicenter, randomized, double-blind, placebo-controlled trial that enrolled 617 patients with persistent, recurrent, or first-line metastatic cervical cancer who had not been treated with chemotherapy except when used concurrently as a radio-sensitizing agent. Patients were enrolled regardless of tumor PD-L1 expression status. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by metastatic status at initial diagnosis, investigator decision to use bevacizumab, and PD-L1 status (CPS <1 vs. CPS 1 to <10 vs. CPS ≥10). Patients were randomized (1:1) to one of the two treatment groups:
- Treatment Group 1: Intravenous pembrolizumab 200 mg plus chemotherapy with or without bevacizumab
- Treatment Group 2: Placebo plus chemotherapy with or without bevacizumab
The investigator selected one of the following four treatment regimens prior to randomization:
- Paclitaxel 175 mg/m2 + cisplatin 50 mg/m2
- Paclitaxel 175 mg/m2 + cisplatin 50 mg/m2 + bevacizumab 15 mg/kg
- Paclitaxel 175 mg/m2 + carboplatin AUC 5 mg/mL/min
- Paclitaxel 175 mg/m2 + carboplatin AUC 5 mg/mL/min + bevacizumab 15 mg/kg
All study medications were administered as an intravenous infusion. All study treatments were administered on Day 1 of each 3-week treatment cycle. Cisplatin could be administered on Day 2 of each 3-week treatment cycle. Treatment with intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease, unacceptable toxicity, or a maximum of 24 months. Administration of intravenous pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed every 9 weeks for the first year, followed by every 12 weeks thereafter. The main efficacy outcome measures were OS and PFS as assessed by investigator according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures were ORR and DoR, according to RECIST v1.1, as assessed by investigator.
Of the 617 enrolled patients, 548 patients (89%) had tumors expressing PD-L1 with a CPS ≥1. Among these 548 enrolled patients with tumors expressing PD-L1, 273 patients were randomized to intravenous pembrolizumab in combination with chemotherapy with or without bevacizumab, and 275 patients were randomized to placebo in combination with chemotherapy with or without bevacizumab. Sixty-three percent of the 548 patients received bevacizumab as part of study treatment. The baseline characteristics of the 548 patients were: median age of 51 years (range: 22 to 82), 16% age 65 or older; 59% White, 18% Asian, 6% American Indian or Alaska Native, and 1% Black; 37% Hispanic or Latino; 56% ECOG performance status 0 and 43% ECOG performance status 1. Seventy-five percent had squamous cell carcinoma, 21% adenocarcinoma, and 5% adenosquamous histology, and 32% of patients had metastatic disease at diagnosis. At study entry, 21% of patients had metastatic disease only and 79% had persistent or recurrent disease with or without distant metastases, of whom 39% had received prior chemoradiation only and 17% had received prior chemoradiation plus surgery.
A statistically significant improvement in OS and PFS was demonstrated in patients randomized to receive intravenous pembrolizumab compared with patients randomized to receive placebo. An updated OS analysis was conducted at the time of final analysis when 354 deaths in the CPS ≥1 population were observed. Table 87 and Figure 30 summarize the key efficacy measures for KEYNOTE-826 for patients with tumors expressing PD-L1 (CPS ≥1).
Table 87: Efficacy Results in Patients with Persistent, Recurrent, or Metastatic Cervical Cancer (CPS ≥1) in KEYNOTE-826 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
and chemotherapy* with or without bevacizumab
n=273Placebo
and chemotherapy* with or without bevacizumab
n=275
+ Denotes ongoing response
NR = not reached- * Chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin)
- † Based on the stratified Cox proportional hazard model
- ‡ p-Value (one-sided) is compared with the allocated alpha of 0.0055 for this interim analysis (with 72% of the planned number of events for final analysis)
- § p-Value (one-sided) is compared with the allocated alpha of 0.0014 for this interim analysis (with 82% of the planned number of events for final analysis)
- ¶ Response: Best objective response as confirmed complete response or partial response
OS Number of patients with event (%) 118 (43.2) 154 (56.0) Median in months (95% CI) NR (19.8, NR) 16.3 (14.5, 19.4) Hazard ratio† (95% CI) 0.64 (0.50, 0.81) p-Value‡ 0.0001 Updated OS Number of patients with event (%) 153 (56.0%) 201 (73.1%) Median in months (95% CI) 28.6 (22.1, 38.0) 16.5 (14.5, 20.0) Hazard ratio† (95% CI) 0.60 (0.49, 0.74) PFS Number of patients with event (%) 157 (57.5) 198 (72.0) Median in months (95% CI) 10.4 (9.7, 12.3) 8.2 (6.3, 8.5) Hazard ratio† (95% CI) 0.62 (0.50, 0.77) p-Value§ < 0.0001 Objective Response Rate ORR¶ (95% CI) 68% (62, 74) 50% (44, 56) Complete response rate 23% 13% Partial response rate 45% 37% Duration of Response Median in months (range) 18.0 (1.3+, 24.2+) 10.4 (1.5+, 22.0+) Figure 30: Kaplan-Meier Curve for Overall Survival in KEYNOTE-826 (CPS ≥1)* ,† - * Treatment arms include intravenous pembrolizumab plus chemotherapy, with or without bevacizumab, versus placebo plus chemotherapy, with or without bevacizumab.
- † Based on the protocol-specified final OS analysis
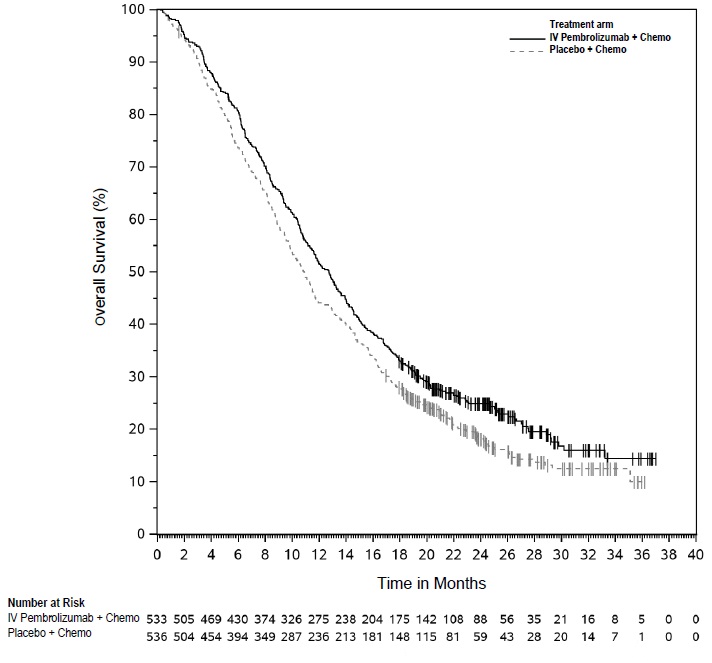
Previously Treated Recurrent or Metastatic Cervical Cancer for Tumors Expressing PD-L1 (CPS≥ 1)
The efficacy of intravenous pembrolizumab was investigated in 98 patients with recurrent or metastatic cervical cancer enrolled in a single cohort (Cohort E) in KEYNOTE-158 (NCT02628067), a multicenter, non-randomized, open-label, multi-cohort trial. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression. Patients received intravenous pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression. Patients with initial radiographic disease progression could receive additional doses of treatment during confirmation of progression unless disease progression was symptomatic, was rapidly progressive, required urgent intervention, or occurred with a decline in performance status. Patients without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks for the first 12 months, and every 12 weeks thereafter. The major efficacy outcome measures were ORR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, as assessed by BICR, and DoR.
Among the 98 patients in Cohort E, 77 (79%) had tumors that expressed PD-L1 with a CPS ≥ 1 and received at least one line of chemotherapy in the metastatic setting. PD-L1 status was determined using the IHC 22C3 pharmDx assay. The baseline characteristics of these 77 patients were: median age of 45 years (range: 27 to 75); 81% White, 14% Asian, and 3% Black; 32% ECOG PS of 0 and 68% ECOG PS of 1; 92% had squamous cell carcinoma, 6% adenocarcinoma, and 1% adenosquamous histology; 95% had M1 disease and 5% had recurrent disease; and 35% had one and 65% had two or more prior lines of therapy in the recurrent or metastatic setting.
No responses were observed in patients whose tumors did not have PD-L1 expression (CPS <1). Efficacy results are summarized in Table 88 for patients with PD-L1 expression (CPS ≥1).
Table 88: Efficacy Results in Patients with Recurrent or Metastatic Cervical Cancer (CPS ≥1) in KEYNOTE-158 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=77*+ Denotes ongoing response
NR = not reached- * Median follow-up time of 11.7 months (range 0.6 to 22.7 months)
- † Based on patients (n=11) with a response by independent review
Objective Response Rate ORR (95% CI) 14.3% (7.4, 24.1) Complete response rate 2.6% Partial response rate 11.7% Duration of Response Median in months (range) NR (4.1, 18.6+)† % with duration ≥6 months 91% 14.12 Hepatocellular Carcinoma
Previously Treated HCC
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-394 (NCT03062358), a multicenter, randomized, placebo-controlled, double-blind trial conducted in Asia in patients with Barcelona Clinic Liver Cancer (BCLC) Stage B or C HCC, who were previously treated with sorafenib or oxaliplatin-based chemotherapy and who were not amenable to or were refractory to local-regional therapy. Patients were also required to have Child-Pugh A liver function.
Patients with hepatitis B had treated controlled disease (HBV viral load <2000 IU/mL or <104 copies/mL). Patients with an autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Patients with hepatic encephalopathy, main branch portal venous invasion, clinically apparent ascites, or esophageal or gastric variceal bleeding within the last 6 months were also ineligible.
Randomization was stratified by prior treatment: sorafenib vs. oxaliplatin-based chemotherapy, macrovascular invasion, and etiology (active HBV vs. others (active HCV, non-infected)). Patients were randomized (2:1) to receive pembrolizumab 200 mg intravenously every 3 weeks or placebo.
Treatment with intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. Assessment of tumor status was performed every 6 weeks. The main efficacy outcome measure was OS. Additional efficacy outcome measures were PFS, ORR, and DoR, as assessed by BICR using RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
The study enrolled 453 patients, and 360 (79%) had active hepatitis B. The population characteristics in patients with active hepatitis B were: median age of 52 years (range: 23 to 82), 16% age 65 or older; 86% male; 100% Asian; 42% ECOG PS of 0 and 58% ECOG PS of 1; 90% received prior sorafenib and 10% received prior oxaliplatin-based chemotherapy. Patient characteristics also included extrahepatic disease (77%), macrovascular invasion (10%), BCLC stage C (93%) and B (7%), and baseline AFP ≥200 ng/mL (57%).
KEYNOTE-394 demonstrated improved OS in patients with HCC secondary to hepatitis B randomized to intravenous pembrolizumab compared with placebo. Efficacy results are summarized in Table 89 and Figure 31.
Table 89: Efficacy Results in Patients with Hepatocellular Carcinoma in KEYNOTE-394 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=236Placebo
n=124+ Denotes ongoing response - * Results at the pre-specified final OS analysis
- † Based on the stratified Cox proportional hazard model
- ‡ Results at pre-specified interim OS analysis
- § Confirmed complete response or partial response
- ¶ Based on Kaplan-Meier estimate
OS* Number (%) of patients with events 172 (73) 105 (85) Median in months (95% CI) 13.9 (12.5, 17.9) 13.0 (10.1, 15.6) Hazard ratio† (95% CI) 0.78 (0.61, 0.99) PFS‡ Number (%) of patients with events 189 (80) 108 (87) Median in months (95% CI) 2 (1.4, 2.7) 2.3 (1.4, 2.8) Hazard ratio† (95% CI) 0.78 (0.61, 1.00) Objective Response Rate‡ ORR§ (95% CI) 11% (7, 16) 1.6% (0.2, 5.7) Number (%) of complete responses 2 (0.9%) 1 (0.8%) Number (%) of partial responses 24 (10%) 1 (0.8%) Duration of Response* n=28 n=2 Median in months¶ (range) 23.9 (2.6+, 44.4+) 5.6 (3.0+, 5.6) Figure 31: Kaplan-Meier Curve for Overall Survival in KEYNOTE-394 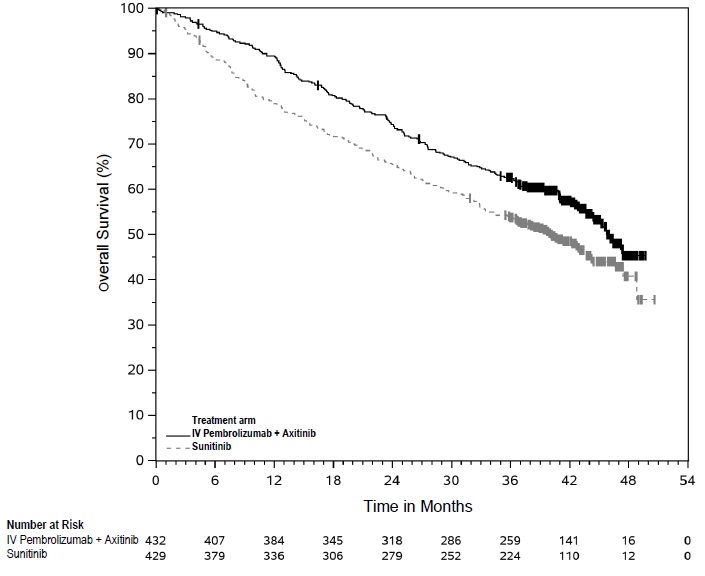
14.13 Biliary Tract Cancer
The efficacy of intravenous pembrolizumab in combination with gemcitabine and cisplatin chemotherapy was investigated in KEYNOTE-966 (NCT04003636), a multicenter, randomized, double-blind, placebo-controlled trial that enrolled 1069 patients with locally advanced unresectable or metastatic BTC, who had not received prior systemic therapy in the advanced disease setting. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by region (Asia vs. non-Asia), locally advanced versus metastatic, and site of origin (gallbladder, intrahepatic or extrahepatic cholangiocarcinoma).
Patients were randomized (1:1) to intravenous pembrolizumab 200 mg on Day 1 plus gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 on Day 1 and Day 8 every 3 weeks, or placebo on Day 1 plus gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 on Day 1 and Day 8 every 3 weeks. Study medications were administered via intravenous infusion. Treatment continued until unacceptable toxicity or disease progression. For pembrolizumab, treatment continued for a maximum of 35 cycles, or approximately 24 months. For gemcitabine, treatment could be continued beyond 8 cycles while for cisplatin, treatment could be administered for a maximum of 8 cycles.
Administration of intravenous pembrolizumab with chemotherapy was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered by the investigator to be deriving clinical benefit. Assessment of tumor status was performed at baseline and then every 6 weeks through 54 weeks, followed by every 12 weeks thereafter.
Study population characteristics were median age of 64 years (range: 23 to 85), 47% age 65 or older; 52% male; 49% White, 46% Asian, 1.3% Black or African American; 10% Hispanic or Latino; 46% ECOG PS of 0 and 54% ECOG PS of 1; 31% of patients had a history of hepatitis B infection, and 3% had a history of hepatitis C infection.
The major efficacy outcome measure was OS. Additional efficacy outcome measures were PFS, ORR and DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
Table 90 and Figure 32 summarize the efficacy results for KEYNOTE-966.
Table 90: Efficacy Results in KEYNOTE-966 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
with
gemcitabine/cisplatin
n=533Placebo with
gemcitabine/cisplatin
n=536NS = not significant - * Results at the pre-specified final OS analysis
- † Based on the stratified Cox proportional hazard model
- ‡ One-sided p-Value based on a stratified log-rank test
- § Results at pre-specified final analysis of PFS and ORR
- ¶ Confirmed complete response or partial response
- # One-sided p-Value based on the stratified Miettinen and Nurminen analysis
- Þ Based on Kaplan-Meier estimate
OS* Number of patients with event (%) 414 (78%) 443 (83%) Median in months (95% CI) 12.7 (11.5, 13.6) 10.9 (9.9, 11.6) Hazard ratio† (95% CI) 0.83 (0.72, 0.95) p-Value‡ 0.0034 PFS§ Number (%) of patients with event 361 (68%) 391 (73%) Median in months (95% CI) 6.5 (5.7, 6.9) 5.6 (5.1, 6.6) Hazard ratio† (95% CI) 0.86 (0.75, 1.00) p-Value‡ NS Objective Response Rate§ ORR¶ (95% CI) 29% (25, 33) 29% (25, 33) Number (%) of complete responses 11 (2.1%) 7 (1.3%) Number (%) of partial responses 142 (27%) 146 (27%) p-Value # NS Duration of Response* n=156 n=152 Median in months Þ (95% CI) 8.3 (6.9, 10.2) 6.8 (5.7, 7.1) Figure 32: Kaplan-Meier Curve for Overall Survival in KEYNOTE-966 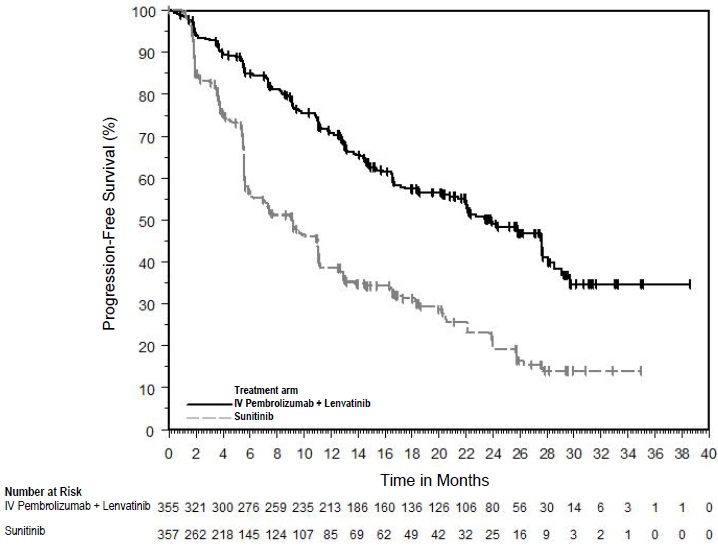
14.14 Merkel Cell Carcinoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-017 (NCT02267603) and KEYNOTE-913 (NCT03783078), two multicenter, non-randomized, open-label trials that enrolled 105 patients with recurrent locally advanced or metastatic MCC who had not received prior systemic therapy for their advanced disease. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible.
Patients received intravenous pembrolizumab 2 mg/kg (KEYNOTE-017) or 200 mg (KEYNOTE-913) every 3 weeks until unacceptable toxicity or disease progression that was symptomatic, rapidly progressive, required urgent intervention, occurred with a decline in performance status, or was confirmed at least 4 weeks later with repeat imaging. Patients without disease progression were treated for up to 24 months.
The major efficacy outcome measures were ORR and DoR as assessed by BICR per RECIST v1.1.
Among the 105 patients enrolled, the median age was 73 years (range: 38 to 91), 79% were age 65 or older; 62% were male; 80% were White, race in 19% was unknown or missing, and 1% were Asian; 53% had ECOG PS of 0, and 47% had ECOG PS of 1. Thirteen percent had stage IIIB disease and 84% had stage IV. Seventy-six percent of patients had prior surgery and 51% had prior radiation therapy.
Efficacy results are summarized in Table 91.
Table 91: Efficacy Results in KEYNOTE-017 and KEYNOTE-913 Endpoint KEYNOTE-017
Intravenous Pembrolizumab
2 mg/kg every
3 weeks
n=50KEYNOTE-913
Intravenous Pembrolizumab
200 mg or 2 mg/kg every
3 weeks
n=55+ Denotes ongoing response NR = not reached Objective Response Rate ORR (95% CI) 56% (41, 70) 49% (35, 63) Complete responses, n (%) 12 (24%) 9 (16%) Partial responses, n (%) 16 (32%) 18 (33%) Duration of Response n=28 n=27 Median DoR in months (range) NR (5.9, 34.5+) NR (4.8, 25.4+) Patients with duration ≥6 months, n (%) 27 (96%) 25 (93%) Patients with duration ≥12 months, n (%) 15 (54%) 19 (70%) 14.15 Renal Cell Carcinoma
First-line treatment with axitinib
KEYNOTE-426
The efficacy of intravenous pembrolizumab in combination with axitinib was investigated in KEYNOTE-426 (NCT02853331), a randomized, multicenter, open-label trial conducted in 861 patients who had not received systemic therapy for advanced RCC. Patients were enrolled regardless of PD-L1 tumor expression status. Patients with active autoimmune disease requiring systemic immunosuppression within the last 2 years were ineligible. Randomization was stratified by International Metastatic RCC Database Consortium (IMDC) risk categories (favorable versus intermediate versus poor) and geographic region (North America versus Western Europe versus “Rest of the World”).
Patients were randomized (1:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg intravenously every 3 weeks up to 24 months in combination with axitinib 5 mg orally, twice daily. Patients who tolerated axitinib 5 mg twice daily for 2 consecutive cycles (6 weeks) could increase to 7 mg and then subsequently to 10 mg twice daily. Axitinib could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- Sunitinib 50 mg orally, once daily for 4 weeks and then off treatment for 2 weeks.
Treatment with intravenous pembrolizumab and axitinib continued until RECIST v1.1-defined progression of disease or unacceptable toxicity. Administration of intravenous pembrolizumab and axitinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at baseline, after randomization at Week 12, then every 6 weeks thereafter until Week 54, and then every 12 weeks thereafter.
The study population characteristics were: median age of 62 years (range: 26 to 90), 38% age 65 or older; 73% male; 79% White and 16% Asian; 20% and 80% of patients had a baseline KPS of 70 to 80 and 90 to 100, respectively; and patient distribution by IMDC risk categories was 31% favorable, 56% intermediate, and 13% poor.
The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures included ORR, as assessed by BICR. A statistically significant improvement in OS was demonstrated at the first pre-specified interim analysis in patients randomized to intravenous pembrolizumab in combination with axitinib compared with sunitinib. The trial also demonstrated statistically significant improvements in PFS and ORR. An updated OS analysis was conducted when 418 deaths were observed based on the planned number of deaths for the pre-specified final analysis. Table 92 and Figure 33 summarize the efficacy results for KEYNOTE-426.
Table 92: Efficacy Results in KEYNOTE-426 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks and Axitinib
n=432Sunitinib
n=429NR = not reached - * Based on the stratified Cox proportional hazard model
- † Based on stratified log-rank test
- ‡ p-Value (one-sided) is compared with the allocated alpha of 0.0001 for this interim analysis (with 39% of the planned number of events for final analysis).
- § p-Value (one-sided) is compared with the allocated alpha of 0.0013 for this interim analysis (with 81% of the planned number of events for final analysis).
- ¶ Response: Best objective response as confirmed complete response or partial response
- # Based on Miettinen and Nurminen method stratified by IMDC risk group and geographic region
OS Number of patients with event (%) 59 (14%) 97 (23%) Median in months (95% CI) NR (NR, NR) NR (NR, NR) Hazard ratio* (95% CI) 0.53 (0.38, 0.74) p-Value† <0.0001‡ Updated OS Number of patients with event (%) 193 (45%) 225 (52%) Median in months (95% CI) 45.7 (43.6, NR) 40.1 (34.3, 44.2) Hazard ratio* (95% CI) 0.73 (0.60, 0.88) PFS Number of patients with event (%) 183 (42%) 213 (50%) Median in months (95% CI) 15.1 (12.6, 17.7) 11.0 (8.7, 12.5) Hazard ratio* (95% CI) 0.69 (0.56, 0.84) p-Value† 0.0001§ Objective Response Rate ORR¶ (95% CI) 59% (54, 64) 36% (31, 40) Complete response rate 6% 2% Partial response rate 53% 34% p-Value# <0.0001 Figure 33: Kaplan-Meier Curve for Updated Overall Survival in KEYNOTE-426 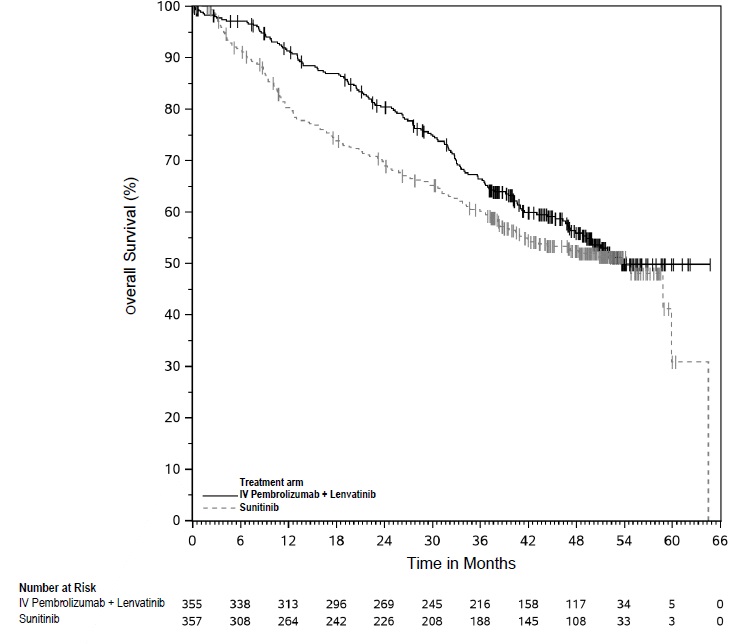
In an exploratory analysis, the updated analysis of OS in patients with IMDC favorable, intermediate, intermediate/poor, and poor risk demonstrated a HR of 1.17 (95% CI: 0.76, 1.80), 0.67 (95% CI: 0.52, 0.86), 0.64 (95% CI: 0.52, 0.80), and 0.51 (95% CI: 0.32, 0.81), respectively.
First-line treatment with lenvatinib
KEYNOTE-581
The efficacy of intravenous pembrolizumab in combination with lenvatinib was investigated in KEYNOTE-581 (NCT02811861), a multicenter, open-label, randomized trial conducted in 1069 patients with advanced RCC in the first-line setting. Patients were enrolled regardless of PD-L1 tumor expression status. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible. Randomization was stratified by geographic region (North America versus Western Europe versus “Rest of the World”) and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic groups (favorable versus intermediate versus poor risk).
Patients were randomized (1:1:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg intravenously every 3 weeks up to 24 months in combination with lenvatinib 20 mg orally once daily.
- Lenvatinib 18 mg orally once daily in combination with everolimus 5 mg orally once daily.
- Sunitinib 50 mg orally once daily for 4 weeks then off treatment for 2 weeks.
Treatment continued until unacceptable toxicity or disease progression. Administration of intravenous pembrolizumab with lenvatinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered by the investigator to be deriving clinical benefit. intravenous pembrolizumab was continued for a maximum of 24 months; however, treatment with lenvatinib could be continued beyond 24 months. Assessment of tumor status was performed at baseline and then every 8 weeks.
The study population characteristics were: median age of 62 years (range: 29 to 88 years), 42% age 65 or older; 75% male; 74% White, 21% Asian, 1% Black, and 2% other races; 18% and 82% of patients had a baseline KPS of 70 to 80 and 90 to 100, respectively; patient distribution by MSKCC risk categories was 27% favorable, 64% intermediate, and 9% poor. Common sites of metastases in patients were lung (68%), lymph node (45%), and bone (25%).
The major efficacy outcome measures were PFS, as assessed by independent radiologic review (IRC) according to RECIST v1.1, and OS. Additional efficacy outcome measures included confirmed ORR as assessed by IRC. intravenous pembrolizumab in combination with lenvatinib demonstrated statistically significant improvements in PFS, OS, and ORR compared with sunitinib. An updated OS analysis was conducted when 304 deaths were observed based on the planned number of deaths for the pre-specified final analysis. Table 93 and Figures 34 and 35 summarize the efficacy results for KEYNOTE-581.
Table 93: Efficacy Results in KEYNOTE-581 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
and Lenvatinib
n=355Sunitinib
n=357Tumor assessments were based on RECIST 1.1; only confirmed responses are included for ORR.
Data cutoff date = 28 Aug 2020, Updated OS cutoff date = 31 July 2022
CI = confidence interval; NR= Not reached- * Hazard ratio is based on a Cox Proportional Hazards Model. Stratified by geographic region and MSKCC prognostic groups.
- † Two-sided p-Value based on stratified log-rank test.
- ‡ Two-sided p-Value based upon CMH test.
Progression-Free Survival (PFS) Number of events, n (%) 160 (45%) 205 (57%) Progressive disease 145 (41%) 196 (55%) Death 15 (4%) 9 (3%) Median PFS in months (95% CI) 23.9 (20.8, 27.7) 9.2 (6.0, 11.0) Hazard ratio* (95% CI) 0.39 (0.32, 0.49) p-Value† <0.0001 Overall Survival (OS) Number of deaths, n (%) 80 (23%) 101 (28%) Median OS in months (95% CI) NR (33.6, NR) NR (NR, NR) Hazard ratio* (95% CI) 0.66 (0.49, 0.88) p-Value† 0.0049 Updated OS Number of deaths, n (%) 149 (42%) 159 (45%) Median OS in months (95% CI) 53.7 (48.7, NR) 54.3 (40.9, NR) Hazard ratio* (95% CI) 0.79 (0.63, 0.99) Objective Response Rate (Confirmed) ORR, n (%) 252 (71%) 129 (36%) (95% CI) (66, 76) (31, 41) Complete response rate 16% 4% Partial response rate 55% 32% p-Value‡ <0.0001 Figure 34: Kaplan-Meier Curve for PFS in KEYNOTE-581 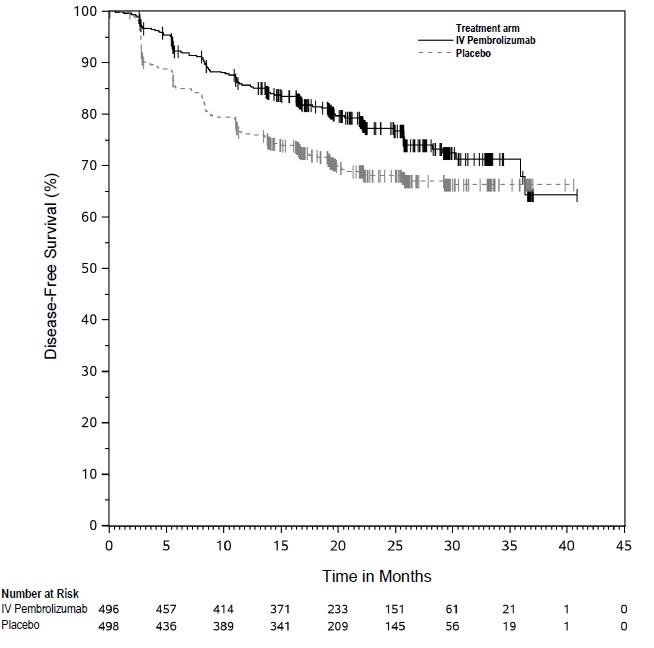
Figure 35: Kaplan-Meier Curve for Updated Overall Survival in KEYNOTE-581 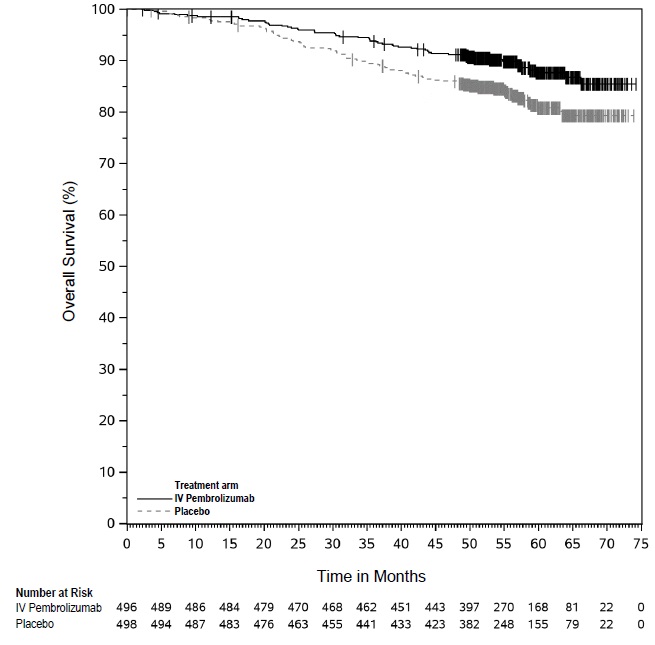
KEYNOTE-B61
The efficacy of intravenous pembrolizumab in combination with lenvatinib was investigated in KEYNOTE-B61 (NCT04704219), a multicenter, single-arm trial that enrolled 160 patients with advanced or metastatic non-clear cell RCC in the first-line setting. Patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible.
Patients received intravenous pembrolizumab 400 mg every 6 weeks in combination with lenvatinib 20 mg orally once daily. Intravenous pembrolizumab was continued for a maximum of 24 months; however, lenvatinib could be continued beyond 24 months. Treatment continued until unacceptable toxicity or disease progression. Administration of intravenous pembrolizumab with lenvatinib was permitted beyond RECIST-defined disease progression if the patient was considered by the investigator to be deriving clinical benefit.
Among the 158 treated patients, the baseline characteristics were: median age of 60 years (range: 24 to 87 years); 71% male; 86% White, 8% Asian, and 3% Black; <1% Hispanic or Latino; 22% and 78% of patients had a baseline KPS of 70 to 80 and 90 to 100, respectively; histologic subtypes were 59% papillary, 18% chromophobe, 4% translocation, <1% medullary, 13% unclassified, and 6% other; patient distribution by IMDC risk categories was 35% favorable, 54% intermediate, and 10% poor. Common sites of metastases in patients were lymph node (65%), lung (35%), bone (30%), and liver (21%).
The major efficacy outcome measure was ORR as assessed by BICR using RECIST 1.1. Additional efficacy outcome measures included DOR as assessed by BICR using RECIST 1.1. Efficacy results are summarized in Table 94.
Table 94: Efficacy Results in KEYNOTE-B61 Endpoint Intravenous Pembrolizumab
400 mg every 6 weeks
and Lenvatinib
n=158CI = confidence interval
+ Denotes ongoing response- * Based on Kaplan-Meier estimates
Objective Response Rate (Confirmed) ORR (95% CI) 51% (43, 59) Complete response 8% Partial response 42% Duration of Response* Median in months (range) 19.5 (1.5+, 23.5+) Adjuvant Treatment of RCC (KEYNOTE-564)
The efficacy of intravenous pembrolizumab was investigated as adjuvant therapy for RCC in KEYNOTE-564 (NCT03142334), a multicenter, randomized (1:1), double-blind, placebo-controlled trial in 994 patients with intermediate-high or high risk of recurrence of RCC, or M1 no evidence of disease (NED). The intermediate-high risk category included: pT2 with Grade 4 or sarcomatoid features; pT3, any Grade without nodal involvement (N0) or distant metastases (M0). The high risk category included: pT4, any Grade N0 and M0; any pT, any Grade with nodal involvement and M0. The M1 NED category included patients with metastatic disease who had undergone complete resection of primary and metastatic lesions. Patients must have undergone a partial nephroprotective or radical complete nephrectomy (and complete resection of solid, isolated, soft tissue metastatic lesion(s) in M1 NED participants) with negative surgical margins ≥4 weeks prior to the time of screening. Patients were excluded from the trial if they had received prior systemic therapy for advanced RCC. Patients with active autoimmune disease or a medical condition that required immunosuppression were also ineligible. Patients were randomized to intravenous pembrolizumab 200 mg administered intravenously every 3 weeks or placebo for up to 1 year until disease recurrence or unacceptable toxicity. Randomization was stratified by metastasis status (M0, M1 NED); M0 group was further stratified by ECOG PS (0,1) and geographic region (US, non-US).
The study population characteristics were: median age of 60 years (range: 25 to 84), 33% age 65 or older; 71% male; 75% White, 14% Asian, 9% Unknown, 1% Black or African American, 1% American Indian or Alaska Native, 1% Multiracial; 13% Hispanic or Latino, 78% Not Hispanic or Latino, 8% Unknown; and 85% ECOG PS of 0 and 15% ECOG PS of 1. Ninety-four percent of patients enrolled had N0 disease; 11% had sarcomatoid features; 86% were intermediate-high risk; 8% were high risk; and 6% were M1 NED. Ninety-two percent of patients had a radical nephrectomy, and 8% had a partial nephrectomy.
The major efficacy outcome measure was investigator-assessed disease-free survival (DFS), defined as time to recurrence, metastasis, or death. An additional outcome measure was OS. Statistically significant improvements in DFS and OS were demonstrated at pre-specified interim analyses in patients randomized to the intravenous pembrolizumab arm compared with placebo. Efficacy results are summarized in Table 95 and Figures 36 and 37.
Table 95: Efficacy Results in KEYNOTE-564 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
n=496Placebo
n=498NR = not reached - * Based on the stratified Cox proportional hazard model
- † Based on stratified log-rank test
- ‡ p-Value (one-sided) is compared with a boundary of 0.0114.
- § p-Value (one-sided) is compared with a boundary of 0.0072.
DFS Number (%) of patients with event 109 (22%) 151 (30%) Median in months (95% CI) NR NR Hazard ratio* (95% CI) 0.68 (0.53, 0.87) p-Value† 0.0010‡ 24-month DFS rate (95% CI) 77% (73, 81) 68% (64, 72) OS Number (%) of patients with event 55 (11%) 86 (17%) Median in months (95% CI) NR (NR, NR) NR (NR, NR) Hazard ratio* (95% CI) 0.62 (0.44, 0.87) p-Value† 0.0024§ 48-month OS rate (95% CI) 91% (88, 93) 86% (83, 89) Figure 36: Kaplan-Meier Curve for Disease-Free Survival in KEYNOTE-564 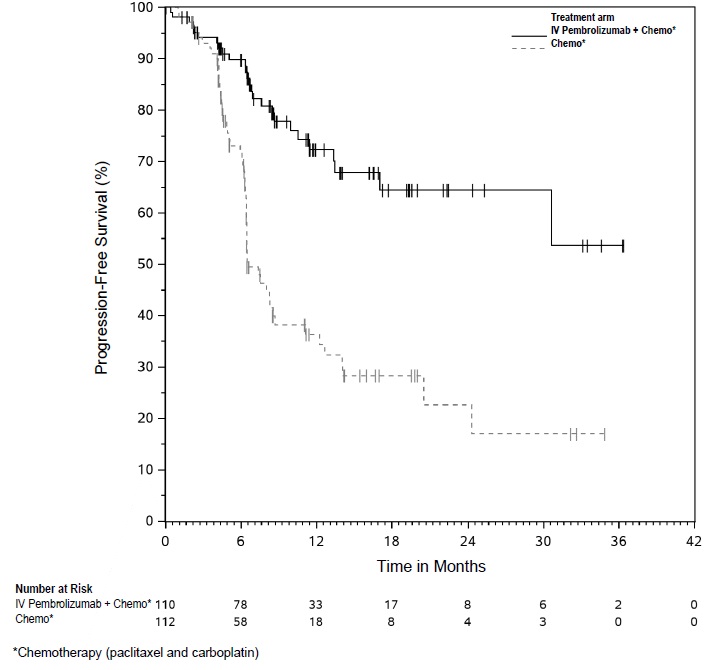
Figure 37: Kaplan-Meier Curve for Overall Survival in KEYNOTE-564 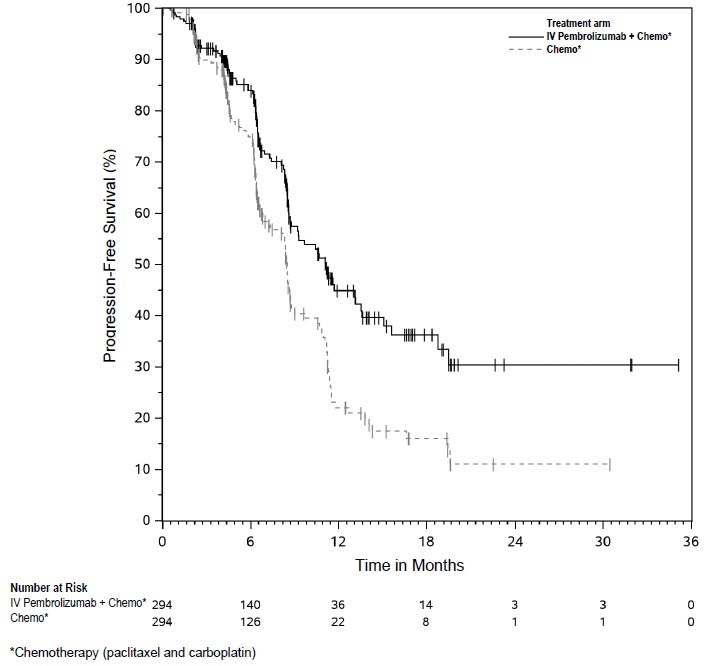
14.16 Endometrial Carcinoma
In Combination with Paclitaxel and Carboplatin for the Treatment of Primary Advanced or Recurrent Endometrial Carcinoma
The efficacy of intravenous pembrolizumab in combination with paclitaxel and carboplatin was investigated in KEYNOTE-868/NRG-GY018 (NCT03914612), a multicenter, randomized, double-blind, placebo-controlled trial in 810 patients with advanced or recurrent endometrial carcinoma. The study design included two separate cohorts based on MMR status; 222 (27%) patients were in dMMR cohort, 588 (73%) patients were in pMMR cohort. The trial enrolled measurable Stage III, measurable Stage IVA, Stage IVB or recurrent endometrial cancer (with or without measurable disease). Patients who had not received prior systemic therapy or had received prior chemotherapy in the adjuvant setting were eligible. Patients who had received prior adjuvant chemotherapy were only eligible if their chemotherapy-free interval was at least 12 months. Patients with endometrial sarcoma, including carcinosarcoma, or patients with active autoimmune disease or a medical condition that required immunosuppression were ineligible. Randomization was stratified according to MMR status, ECOG PS (0 or 1 vs. 2), and prior adjuvant chemotherapy.
Patients were randomized (1:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg every 3 weeks, paclitaxel 175 mg/m2 and carboplatin AUC 5 mg/mL/min for 6 cycles, followed by intravenous pembrolizumab 400 mg every 6 weeks for up to 14 cycles.
- Placebo every 3 weeks, paclitaxel 175 mg/m2 and carboplatin AUC 5 mg/mL/min for 6 cycles, followed by placebo every 6 weeks for up to 14 cycles.
All study medications were administered as an intravenous infusion on Day 1 of each treatment cycle. Treatment continued until disease progression, unacceptable toxicity, or a maximum of 20 cycles (up to approximately 24 months). Patients with measurable disease who had RECIST-defined stable disease or partial response at the completion of cycle 6 were permitted to continue receiving paclitaxel and carboplatin with intravenous pembrolizumab or placebo for up to 10 cycles as determined by the investigator. Assessment of tumor status was performed every 9 weeks for the first 9 months and then every 12 weeks thereafter. The major efficacy outcome measure was PFS as assessed by the investigator according to RECIST 1.1. An additional efficacy outcome measure was OS.
The dMMR population characteristics were: median age of 66 years (range: 37 to 86), 55% age 65 or older; 79% White, 9% Black, and 3% Asian; 5% Hispanic or Latino; 64% ECOG PS of 0, 33% ECOG PS of 1, and 3% ECOG PS of 2; 61% had recurrent disease and 39% had primary or persistent disease; 5% received prior adjuvant chemotherapy and 43% received prior radiotherapy. The histologic subtypes were endometrioid carcinoma (81%), adenocarcinoma NOS (11%), serous carcinoma (2%), and other (6%).
The pMMR population characteristics were: median age of 66 years (range: 29 to 94), 54% age 65 or older; 72% White, 16% Black, and 5% Asian; 6% Hispanic or Latino; 67% ECOG PS of 0, 30% ECOG PS of 1, and 3% ECOG PS of 2; 56% had recurrent disease and 44% had primary or persistent disease; 26% received prior adjuvant chemotherapy and 41% received prior radiotherapy. The histologic subtypes were endometrioid carcinoma (52%), serous carcinoma (26%), adenocarcinoma NOS (10%), clear cell carcinoma (7%), and other (5%).
The trial demonstrated statistically significant improvements in PFS for patients randomized to intravenous pembrolizumab in combination with paclitaxel and carboplatin compared to placebo in combination with paclitaxel and carboplatin in both the dMMR and pMMR populations. Table 96 and Figures 38 and 39 summarize the efficacy results for KEYNOTE-868 by MMR status. At the time of the PFS analysis, OS data were not mature with 12% deaths in the dMMR population and 17% deaths in the pMMR population.
Table 96: Efficacy Results in KEYNOTE-868 Endpoint dMMR Population pMMR Population Intravenous Pembrolizumab
with paclitaxel and
carboplatin
n=110Placebo
with paclitaxel and
carboplatin
n=112Intravenous Pembrolizumab
with paclitaxel and
carboplatin
n=294Placebo
with paclitaxel and
carboplatin
n=294NR = not reached - * Based on interim PFS analysis; the information fractions for interim analyses were 49% for dMMR and 55% for pMMR.
- † Based on the stratified Cox proportional hazard model
- ‡ Based on the stratified log-rank test
PFS* Number (%) of patients with event 26 (24%) 57 (51%) 91 (31%) 124 (42%) Median in months (95% CI) NR (30.7, NR) 6.5 (6.4, 8.7) 11.1 (8.7, 13.5) 8.5 (7.2, 8.8) Hazard ratio† (95% CI) 0.30 (0.19, 0.48) 0.60 (0.46, 0.78) p-Value‡ <0.0001 <0.0001 Figure 38: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-868 (dMMR Population) 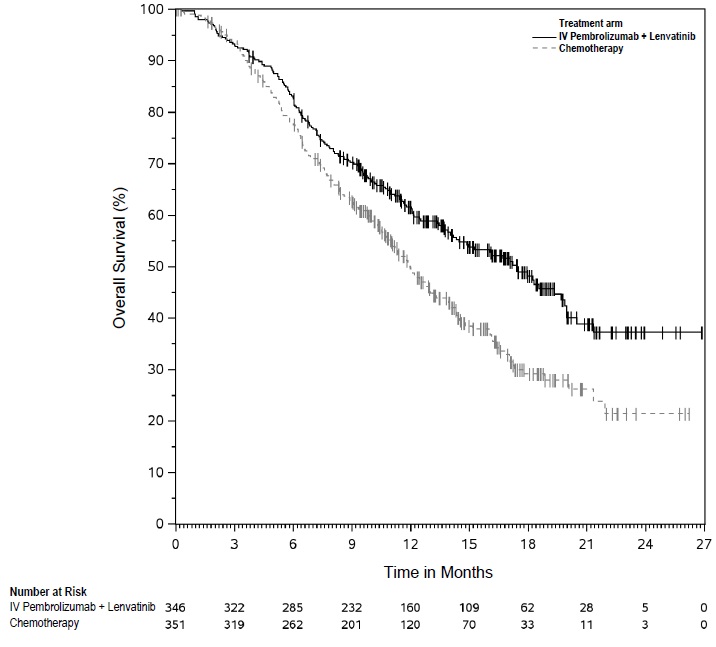
Figure 39: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-868 (pMMR Population) 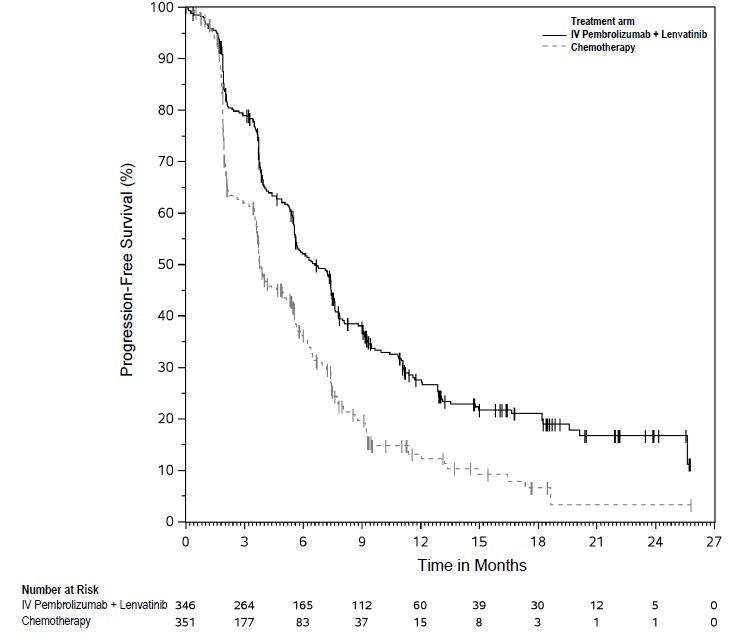
Lack of Effectiveness for Adjuvant Treatment of Patients with Endometrial Carcinoma
The efficacy of intravenous pembrolizumab in combination with carboplatin and paclitaxel, with or without radiation, was investigated in KEYNOTE‑B21 (NCT04634877), a randomized, multicenter, double-blind, placebo-controlled trial in 1,095 patients with newly-diagnosed, high‑risk endometrial cancer with no evidence of disease on imaging following curative intent surgery. High-risk disease was defined as any of the following (staging per FIGO 2009): Stage I/II with myometrial invasion and either non-endometrioid histology or aberrant p53 expression or p53 mutation, or Stage III/IVA. The trial did not meet the prespecified primary endpoint for investigator-assessed DFS, with a HR of 1.02 (95% CI: 0.79, 1.32).
In Combination with Lenvatinib for the Treatment of Advanced Endometrial Carcinoma That Is pMMR or Not MSI-H
The efficacy of intravenous pembrolizumab in combination with lenvatinib was investigated in KEYNOTE-775 (NCT03517449), a multicenter, open-label, randomized, active-controlled trial that enrolled 827 patients with advanced endometrial carcinoma who had been previously treated with at least one prior platinum-based chemotherapy regimen in any setting, including in the neoadjuvant and adjuvant settings. Patients with endometrial sarcoma, including carcinosarcoma, or patients who had active autoimmune disease or a medical condition that required immunosuppression were ineligible. Patients with endometrial carcinoma that were pMMR (using the VENTANA MMR RxDx Panel test) or not MSI-H were stratified by ECOG performance status, geographic region, and history of pelvic radiation. Patients were randomized (1:1) to one of the following treatment arms:
- Intravenous pembrolizumab 200 mg intravenously every 3 weeks in combination with lenvatinib 20 mg orally once daily.
- Investigator’s choice, consisting of either doxorubicin 60 mg/m2 every 3 weeks or paclitaxel 80 mg/m2 given weekly, 3 weeks on/1 week off.
Treatment with intravenous pembrolizumab and lenvatinib continued until RECIST v1.1-defined progression of disease as verified by BICR, unacceptable toxicity, or for intravenous pembrolizumab, a maximum of 24 months. Treatment was permitted beyond RECIST v1.1-defined disease progression if the treating investigator considered the patient to be deriving clinical benefit, and the treatment was tolerated. Assessment of tumor status was performed every 8 weeks. The major efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ. Additional efficacy outcome measures included ORR and DoR, as assessed by BICR.
Among the 697 pMMR patients, 346 patients were randomized to intravenous pembrolizumab in combination with lenvatinib, and 351 patients were randomized to investigator’s choice of doxorubicin (n=254) or paclitaxel (n=97). The pMMR population characteristics were: median age of 65 years (range: 30 to 86), 52% age 65 or older; 62% White, 22% Asian, and 3% Black; 60% ECOG PS of 0 and 40% ECOG PS of 1. The histologic subtypes were endometrioid carcinoma (55%), serous (30%), clear cell carcinoma (7%), mixed (4%), and other (3%). All 697 of these patients received prior systemic therapy for endometrial carcinoma: 67% had one, 30% had two, and 3% had three or more prior systemic therapies. Thirty-seven percent of patients received only prior neoadjuvant or adjuvant therapy.
Efficacy results for the pMMR or not MSI-H patients are summarized in Table 97 and Figures 40 and 41.
Table 97: Efficacy Results in KEYNOTE-775 Endometrial Carcinoma (pMMR or not MSI-H) Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
and Lenvatinib
n=346
Doxorubicin or Paclitaxel
n=351- * Based on the stratified Cox regression model
- † Based on stratified log-rank test
- ‡ Response: Best objective response as confirmed complete response or partial response
- § Based on Miettinen and Nurminen method stratified by ECOG performance status, geographic region, and history of pelvic radiation
OS Number (%) of patients with event 165 (48%) 203 (58%) Median in months (95% CI) 17.4 (14.2, 19.9) 12.0 (10.8, 13.3) Hazard ratio* (95% CI) 0.68 (0.56, 0.84) p-Value† 0.0001 PFS Number (%) of patients with event 247 (71%) 238 (68%) Median in months (95% CI) 6.6 (5.6, 7.4) 3.8 (3.6, 5.0) Hazard ratio* (95% CI) 0.60 (0.50, 0.72) p-Value† <0.0001 Objective Response Rate ORR‡ (95% CI) 30% (26, 36) 15% (12, 19) Complete response rate 5% 3% Partial response rate 25% 13% p-Value§ <0.0001 Duration of Response n=105 n=53 Median in months (range) 9.2 (1.6+, 23.7+) 5.7 (0.0+, 24.2+) Figure 40: Kaplan-Meier Curve for Overall Survival in KEYNOTE-775 (pMMR or Not MSI-H) 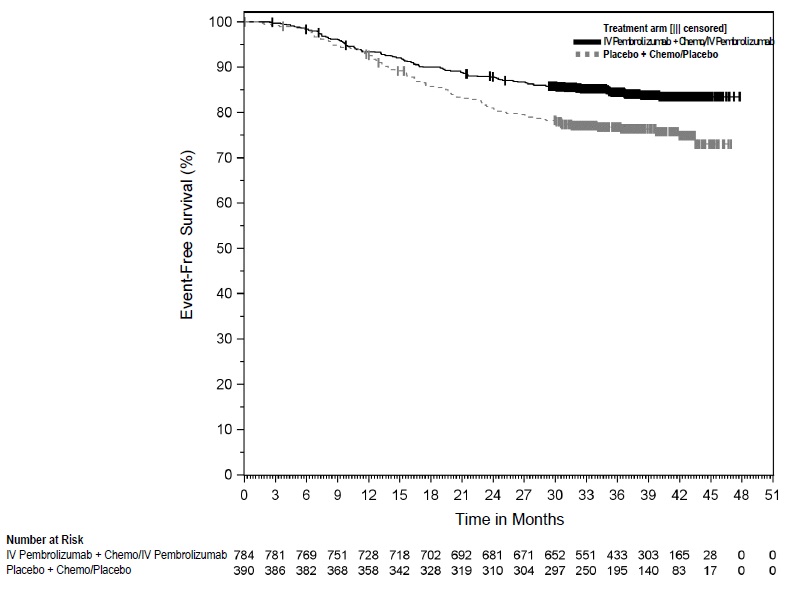
Figure 41: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-775 (pMMR or Not MSI-H) 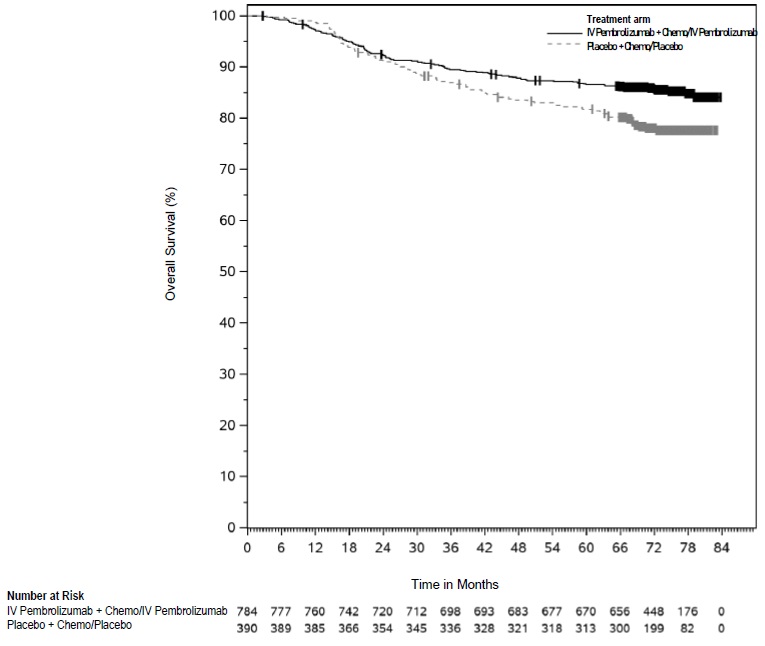
As a Single Agent for the Treatment of Advanced MSI-H or dMMR Endometrial Carcinoma
The efficacy of intravenous pembrolizumab was investigated in KEYNOTE-158 (NCT02628067), a multicenter, non-randomized, open-label, multi-cohort trial. The trial enrolled 90 patients with unresectable or metastatic MSI-H or dMMR endometrial carcinoma in Cohorts D and K. MSI or MMR tumor status was determined using polymerase chain reaction (PCR) or immunohistochemistry (IHC), respectively. Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Patients received intravenous pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression. Patients treated with intravenous pembrolizumab without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks for the first 12 months, and every 12 weeks thereafter. The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
Among the 90 patients evaluated, the baseline characteristics were: median age of 64 years (range: 42 to 86); 83% White, 8% Asian, and 3% Black; 12% Hispanic or Latino; 39% ECOG PS of 0 and 61% ECOG PS of 1; 96% had M1 disease and 4% had M0 disease at study entry; and 51% had one and 48% had two or more prior lines of therapy. Nine patients received only adjuvant therapy and one patient received only neoadjuvant and adjuvant therapy before participating in the study.
Efficacy results are summarized in Table 98.
Table 98: Efficacy Results in Patients with Advanced MSI-H or dMMR Endometrial Carcinoma in KEYNOTE-158 Endpoint Intravenous Pembrolizumab
n=90*+ Denotes ongoing response
NR = not reached- * Median follow-up time of 16.0 months (range 0.5 to 62.1 months)
Objective Response Rate ORR (95% CI) 46% (35, 56) Complete response rate 12% Partial response rate 33% Duration of Response n=41 Median in months (range) NR (2.9, 55.7+) % with duration ≥12 months 68% % with duration ≥24 months 44% 14.17 Tumor Mutational Burden-High Cancer
The efficacy of intravenous pembrolizumab was investigated in a prospectively-planned retrospective analysis of 10 cohorts (A through J) of patients with various previously treated unresectable or metastatic solid tumors with high tumor mutation burden (TMB-H) who were enrolled in a multicenter, non-randomized, open-label trial, KEYNOTE-158 (NCT02628067). The trial excluded patients who previously received an anti-PD-1 or other immune-modulating monoclonal antibody, or who had an autoimmune disease, or a medical condition that required immunosuppression. Patients received intravenous pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression. Assessment of tumor status was performed every 9 weeks for the first 12 months and every 12 weeks thereafter.
The statistical analysis plan pre-specified ≥10 and ≥13 mutations per megabase using the FoundationOne CDx assay as cutpoints to assess TMB. Testing of TMB was blinded with respect to clinical outcomes. The major efficacy outcome measures were ORR and DoR in patients who received at least one dose of intravenous pembrolizumab as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
In KEYNOTE-158, 1050 patients were included in the efficacy analysis population. TMB was analyzed in the subset of 790 patients with sufficient tissue for testing based on protocol-specified testing requirements. Of the 790 patients, 102 (13%) had tumors identified as TMB-H, defined as TMB ≥10 mutations per megabase. Among the 102 patients with TMB-H advanced solid tumors, the study population characteristics were: median age of 61 years (range: 27 to 80), 34% age 65 or older; 34% male; 81% White; and 41% ECOG PS of 0 and 58% ECOG PS of 1. Fifty-six percent of patients had at least two prior lines of therapy.
Efficacy results are summarized in Tables 99 and 100.
Table 99: Efficacy Results for Patients with TMB-H Cancer in KEYNOTE-158
EndpointIntravenous Pembrolizumab
200 mg every 3 weeksTMB ≥10 mut/Mb
n=102*TMB ≥13 mut/Mb
n=70+ Denotes ongoing response
NR = not reached- * Median follow-up time of 11.1 months
- † From product-limit (Kaplan-Meier) method for censored data
Objective Response Rate ORR (95% CI) 29% (21, 39) 37% (26, 50) Complete response rate 4% 3% Partial response rate 25% 34% Duration of Response n=30 n=26 Median in months (range)† NR (2.2+, 34.8+) NR (2.2+, 34.8+) % with duration ≥12 months 57% 58% % with duration ≥24 months 50% 50% Table 100: Response by Tumor Type (TMB ≥10 mut/Mb) Objective Response Rate Duration of Response range N n (%) 95% CI (months) CR = complete response
PR = partial response
SD = stable disease
PD = progressive disease- * No TMB-H patients were identified in the cholangiocarcinoma cohort
Overall* 102 30 (29%) (21%, 39%) (2.2+, 34.8+) Small cell lung cancer 34 10 (29%) (15%, 47%) (4.1, 32.5+) Cervical cancer 16 5 (31%) (11%, 59%) (3.7+, 34.8+) Endometrial cancer 15 7 (47%) (21%, 73%) (8.4+, 33.9+) Anal cancer 14 1 (7%) (0.2%, 34%) 18.8+ Vulvar cancer 12 2 (17%) (2%, 48%) (8.8, 11.0) Neuroendocrine cancer 5 2 (40%) (5%, 85%) (2.2+, 32.6+) Salivary cancer 3 PR, SD, PD 31.3+ Thyroid cancer 2 CR, CR (8.2, 33.2+) Mesothelioma cancer 1 PD In an exploratory analysis in 32 patients enrolled in KEYNOTE-158 whose cancer had TMB ≥10 mut/Mb and <13 mut/Mb, the ORR was 13% (95% CI: 4%, 29%), including two complete responses and two partial responses.
14.18 Cutaneous Squamous Cell Carcinoma
The efficacy of intravenous pembrolizumab was investigated in patients with recurrent or metastatic cSCC or locally advanced cSCC enrolled in KEYNOTE-629 (NCT03284424), a multicenter, multi-cohort, non-randomized, open-label trial. The trial excluded patients with autoimmune disease or a medical condition that required immunosuppression.
Patients received intravenous pembrolizumab 200 mg intravenously every 3 weeks until documented disease progression, unacceptable toxicity, or a maximum of 24 months. Patients with initial radiographic disease progression could receive additional doses of intravenous pembrolizumab during confirmation of progression unless disease progression was symptomatic, rapidly progressive, required urgent intervention, or occurred with a decline in performance status.
Assessment of tumor status was performed every 6 weeks during the first year, and every 9 weeks during the second year. The major efficacy outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ.
Among the 105 patients with recurrent or metastatic cSCC treated, the study population characteristics were: median age of 72 years (range: 29 to 95), 71% age 65 or older; 76% male; 70% White, 25% race unknown; 34% ECOG PS of 0 and 66% ECOG PS of 1. Forty-five percent of patients had locally recurrent only cSCC, 24% had metastatic only cSCC, and 31% had both locally recurrent and metastatic cSCC. Eighty-seven percent received one or more prior lines of therapy; 73% received prior radiation therapy.
Among the 54 patients with locally advanced cSCC treated, the study population characteristics were: median age of 76 years (range: 35 to 95), 80% age 65 or older; 72% male; 83% White, 13% race unknown; 41% ECOG PS of 0 and 59% ECOG PS of 1. Twenty-two percent received one or more prior lines of therapy; 63% received prior radiation therapy.
Efficacy results are summarized in Table 101.
Table 101: Efficacy Results in KEYNOTE-629 Endpoint Intravenous Pembrolizumab
Recurrent or Metastatic cSCC
n=105Intravenous Pembrolizumab
Locally Advanced cSCC
n=54+ Denotes ongoing response - * Median follow-up time: recurrent or metastatic cSCC: 23.8 months; locally advanced cSCC: 48.0 months
Objective Response Rate ORR (95% CI) 35% (26, 45) 52% (38, 66) Complete response rate 12% 22% Partial response rate 23% 30% Duration of Response* n=37 n=28 Median in months (range) NR (2.7, 64.2+) 47.2 (1.0+, 49.9+) % with duration ≥6 months 76% 89% % with duration ≥12 months 68% 75% 14.19 Triple-Negative Breast Cancer
Neoadjuvant and Adjuvant Treatment of High-Risk Early-Stage TNBC
The efficacy of intravenous pembrolizumab in combination with neoadjuvant chemotherapy followed by surgery and continued adjuvant treatment with intravenous pembrolizumab as a single agent was investigated in KEYNOTE-522 (NCT03036488), a randomized (2:1), multicenter, double-blind, placebo-controlled trial conducted in 1174 patients with newly diagnosed previously untreated high-risk early-stage TNBC (tumor size >1 cm but ≤2 cm in diameter with nodal involvement or tumor size >2 cm in diameter regardless of nodal involvement). Patients were enrolled regardless of tumor PD-L1 expression. Patients with active autoimmune disease that required systemic therapy within two years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by nodal status (positive vs. negative), tumor size (T1/T2 vs. T3/T4), and choice of carboplatin (dosed every 3 weeks vs. weekly).
Patients were randomized (2:1) to one of the following two treatment arms; all study medications were administered intravenously:
-
Arm 1:
- Four cycles of preoperative intravenous pembrolizumab 200 mg every 3 weeks on Day 1 of cycles 1-4 of treatment regimen in combination with:
- Carboplatin
- AUC 5 mg/mL/min every 3 weeks on Day 1 of cycles 1-4 of treatment regimen
-or- - AUC 1.5 mg/mL/min every week on Days 1, 8, and 15 of cycles 1-4 of treatment regimen
-and-
- AUC 5 mg/mL/min every 3 weeks on Day 1 of cycles 1-4 of treatment regimen
- Paclitaxel 80 mg/m2 every week on Days 1, 8, and 15 of cycles 1-4 of treatment regimen
- Carboplatin
- Followed by four additional cycles of preoperative intravenous pembrolizumab 200 mg every 3 weeks on Day 1 of cycles 5-8 of treatment regimen in combination with:
- Doxorubicin 60 mg/m2 -or- epirubicin 90 mg/m2 every 3 weeks on Day 1 of cycles 5-8 of treatment regimen -and-
- Cyclophosphamide 600 mg/m2 every 3 weeks on Day 1 of cycles 5-8 of treatment regimen
- Following surgery, nine cycles of intravenous pembrolizumab 200 mg every 3 weeks were administered.
- Four cycles of preoperative intravenous pembrolizumab 200 mg every 3 weeks on Day 1 of cycles 1-4 of treatment regimen in combination with:
-
Arm 2:
- Four cycles of preoperative placebo every 3 weeks on Day 1 of cycles 1-4 of treatment regimen in combination with:
- Carboplatin
- AUC 5 mg/mL/min every 3 weeks on Day 1 of cycles 1-4 of treatment regimen
-or- - AUC 1.5 mg/mL/min every week on Days 1, 8, and 15 of cycles 1-4 of treatment regimen
-and-
- AUC 5 mg/mL/min every 3 weeks on Day 1 of cycles 1-4 of treatment regimen
- Paclitaxel 80 mg/m2 every week on Days 1, 8, and 15 of cycles 1-4 of treatment regimen
- Carboplatin
- Followed by four cycles of preoperative placebo every 3 weeks on Day 1 of cycles 5-8 of treatment regimen in combination with:
- Doxorubicin 60 mg/m2 -or- epirubicin 90 mg/m2 every 3 weeks on Day 1 of cycles 5-8 of treatment regimen -and-
- Cyclophosphamide 600 mg/m2 every 3 weeks on Day 1 of cycles 5-8 of treatment regimen
- Following surgery, nine cycles of placebo every 3 weeks were administered.
- Four cycles of preoperative placebo every 3 weeks on Day 1 of cycles 1-4 of treatment regimen in combination with:
The trial was not designed to isolate the effect of intravenous pembrolizumab in each phase (neoadjuvant or adjuvant) of treatment.
The main efficacy outcomes were pCR rate and EFS. pCR was defined as absence of invasive cancer in the breast and lymph nodes (ypT0/Tis ypN0) and was assessed by the blinded local pathologist at the time of definitive surgery. EFS was defined as the time from randomization to the first occurrence of any of the following events: progression of disease that precludes definitive surgery, local or distant recurrence, second primary malignancy, or death due to any cause. An additional efficacy outcome was overall survival (OS).
The study population characteristics were: median age of 49 years (range: 22 to 80), 11% age 65 or older; 99.9% female; 64% White, 20% Asian, 4.5% Black, and 1.8% American Indian or Alaska Native; 87% ECOG PS of 0 and 13% ECOG PS of 1; 56% were pre-menopausal status and 44% were post-menopausal status; 7% were primary Tumor 1 (T1), 68% T2, 19% T3, and 7% T4; 49% were nodal involvement 0 (N0), 40% N1, 11% N2, and 0.2% N3; 75% of patients were overall Stage II and 25% were Stage III.
Statistically significant improvements in pCR, EFS, and OS were demonstrated at pre-specified interim analyses for patients randomized to intravenous pembrolizumab in combination with chemotherapy followed by intravenous pembrolizumab as a single agent compared with patients randomized to placebo in combination with chemotherapy followed by placebo alone.
Table 102 and Figures 42 and 43 summarize the efficacy results for KEYNOTE-522.
Table 102: Efficacy Results in KEYNOTE-522 Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
with chemotherapy/Intravenous Pembrolizumab
n=784Placebo
with chemotherapy/Placebo
n=390- * Based on the entire intention-to-treat population n=1174 patients
- † Based on a pre-specified pCR interim analysis in n=602 patients, the pCR rate difference was statistically significant (p=0.00055 compared to a significance level of 0.003).
- ‡ Based on Miettinen and Nurminen method stratified by nodal status, tumor size, and choice of carboplatin
- § Based on stratified Cox regression model
- ¶ Based on a pre-specified EFS interim analysis (compared to a significance level of 0.0052)
- # Based on log-rank test stratified by nodal status, tumor size, and choice of carboplatin
- Þ Based on a pre-specified OS interim analysis (compared to a significance level of 0.0050)
pCR (ypT0/Tis ypN0)* Number of patients with pCR 494 217 pCR rate (%), (95% CI) 63.0 (59.5, 66.4) 55.6 (50.6, 60.6) Treatment difference (%) estimate (95% CI)†,‡ 7.5 (1.6, 13.4) EFS Number of patients with event (%) 123 (16%) 93 (24%) Hazard ratio (95% CI)§ 0.63 (0.48, 0.82) p-Value¶,# 0.00031 OS Number of patients with event (%) 115 (15%) 85 (22%) Hazard ratio (95% CI)§ 0.66 (0.50, 0.87) p-Value#,Þ 0.00150 Figure 42: Kaplan-Meier Curve for Event-Free Survival in KEYNOTE-522 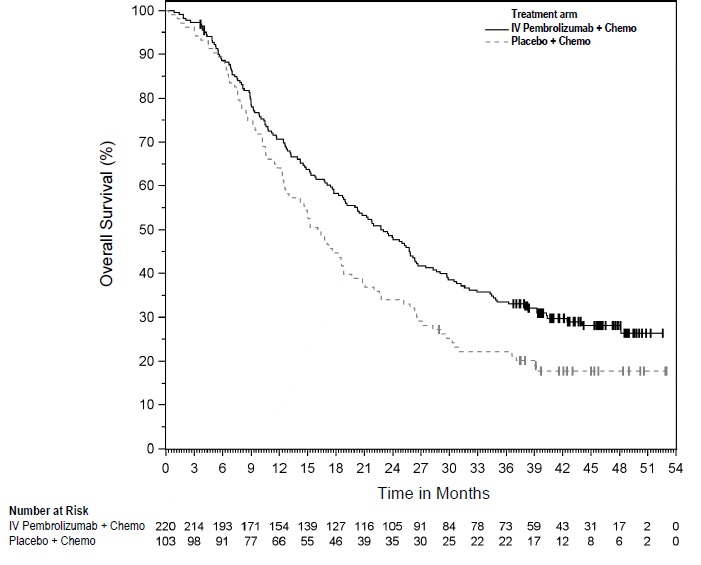
Figure 43: Kaplan-Meier Curve for Overall Survival in KEYNOTE-522 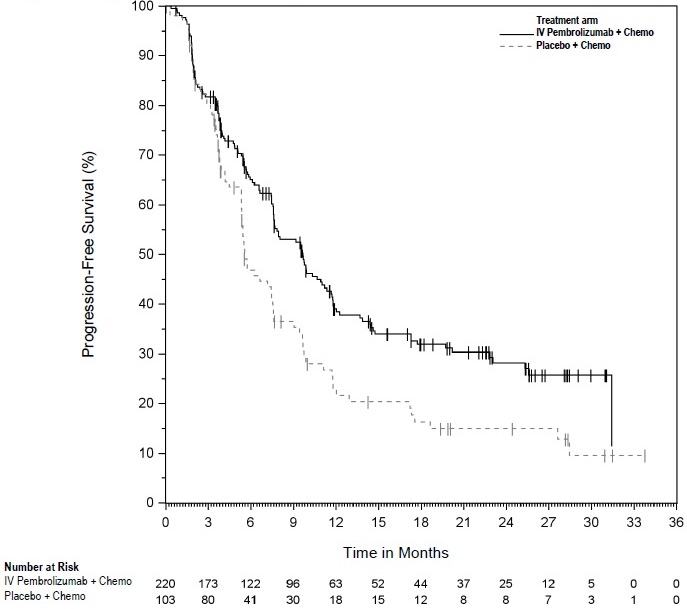
Locally Recurrent Unresectable or Metastatic TNBC for Tumors Expressing PD-L1 (CPS≥ 10)
The efficacy of intravenous pembrolizumab in combination with paclitaxel, paclitaxel protein-bound, or gemcitabine and carboplatin was investigated in KEYNOTE-355 (NCT02819518), a multicenter, double-blind, randomized, placebo-controlled trial conducted in 847 patients with locally recurrent unresectable or metastatic TNBC, regardless of tumor PD-L1 expression, who had not been previously treated with chemotherapy in the metastatic setting. Patients with active autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by chemotherapy treatment (paclitaxel or paclitaxel protein-bound vs. gemcitabine and carboplatin), tumor PD-L1 expression (CPS ≥1 vs. CPS <1) according to the PD-L1 IHC 22C3 pharmDx assay, and prior treatment with the same class of chemotherapy in the neoadjuvant setting (yes vs. no).
Patients were randomized (2:1) to one of the following treatment arms; all study medications were administered via intravenous infusion:
- Intravenous pembrolizumab 200 mg on Day 1 every 3 weeks in combination with paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 every 28 days, paclitaxel 90 mg/m2 on Days 1, 8, and 15 every 28 days, or gemcitabine 1000 mg/m2 and carboplatin AUC 2 mg/mL/min on Days 1 and 8 every 21 days.
- Placebo on Day 1 every 3 weeks in combination with paclitaxel protein-bound 100 mg/m2 on Days 1, 8 and 15 every 28 days, paclitaxel 90 mg/m2 on Days 1, 8, and 15 every 28 days, or gemcitabine 1000 mg/m2 and carboplatin AUC 2 mg/mL/min on Days 1 and 8 every 21 days.
Assessment of tumor status was performed at Weeks 8, 16, and 24, then every 9 weeks for the first year, and every 12 weeks thereafter. The main efficacy outcome measures were OS and PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, tested in the subgroup of patients with CPS ≥10. Additional efficacy outcome measures were ORR and DoR as assessed by BICR.
The study population characteristics for patients were: median age of 53 years (range: 22 to 85), 21% age 65 or older; 100% female; 68% White, 21% Asian, and 4% Black; 60% ECOG PS of 0 and 40% ECOG PS of 1; and 68% were post-menopausal status. Seventy-five percent of patients had tumor PD-L1 expression CPS ≥1 and 38% had tumor PD-L1 expression CPS ≥10.
Table 103 and Figures 44 and 45 summarize the efficacy results for KEYNOTE-355.
Table 103: Efficacy Results in KEYNOTE-355 (CPS ≥10) Endpoint Intravenous Pembrolizumab
200 mg every 3 weeks
with chemotherapy
n=220Placebo
every 3 weeks
with chemotherapy
n=103- * Based on the pre-specified final analysis
- † Based on stratified Cox regression model
- ‡ One-sided p-Value based on stratified log-rank test (compared to a significance level of 0.0113)
- § Based on a pre-specified interim analysis
- ¶ One-sided p-Value based on stratified log-rank test (compared to a significance level of 0.00411)
OS* Number of patients with event (%) 155 (70%) 84 (82%) Median in months (95% CI) 23 (19.0, 26.3) 16.1 (12.6, 18.8) Hazard ratio† (95% CI) 0.73 (0.55, 0.95) p-Value‡ 0.0093 PFS§ Number of patients with event
(%)136 (62%) 79 (77%) Median in months (95% CI) 9.7 (7.6, 11.3) 5.6 (5.3, 7.5) Hazard ratio† (95% CI) 0.65 (0.49, 0.86) p-Value¶ 0.0012 Objective Response Rate (Confirmed)* ORR (95% CI) 53% (46, 59) 41% (31, 51) Complete response rate 17% 14% Partial response rate 35% 27% Duration of Response* n=116 n=42 Median in months (95% CI) 12.8 (9.9, 25.9) 7.3 (5.5, 15.4) Figure 44: Kaplan-Meier Curve for Overall Survival in KEYNOTE-355 (CPS ≥10) 
Figure 45: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-355 (CPS ≥10) 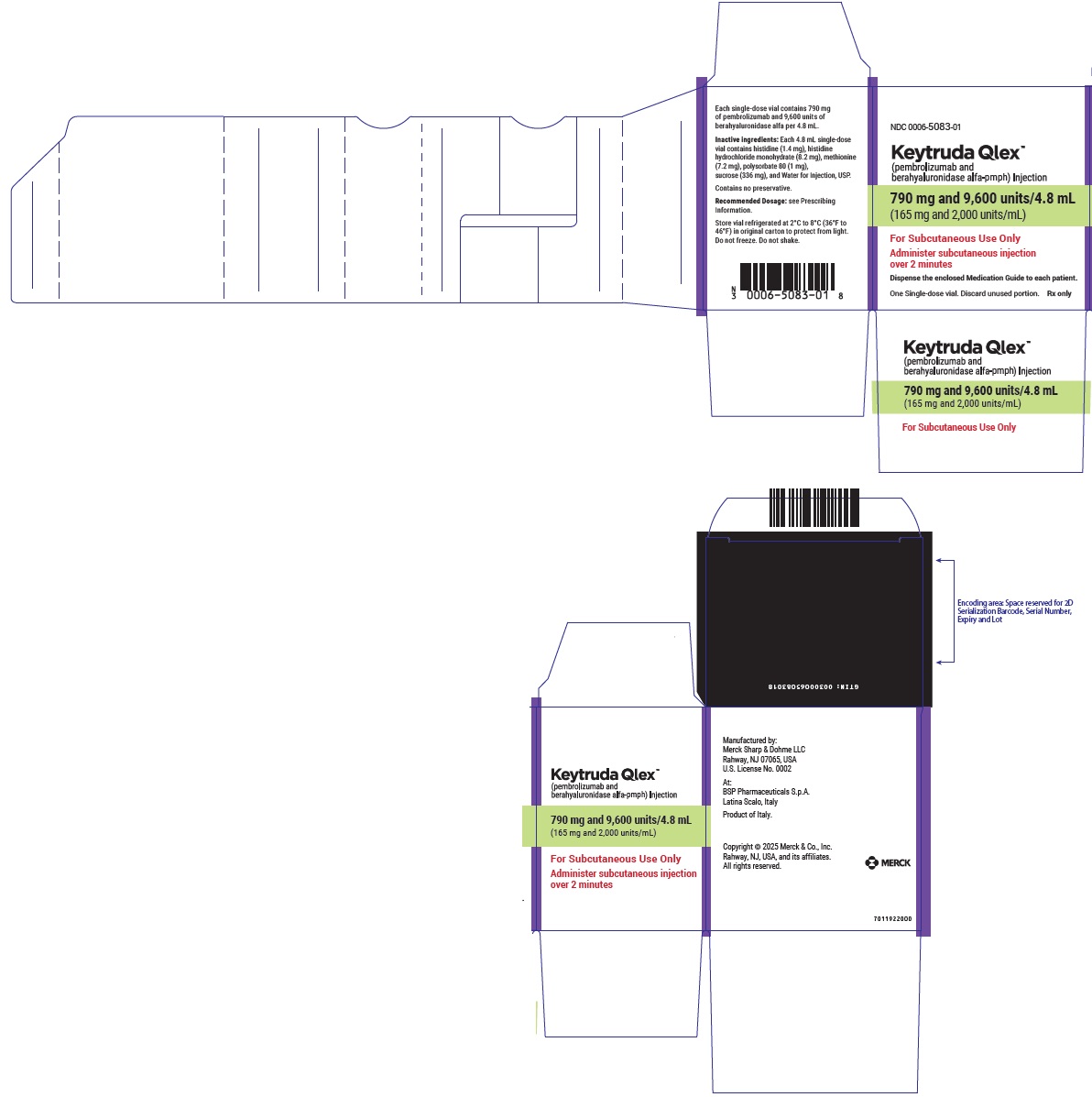
14.20 Ovarian Cancer
The efficacy of intravenous pembrolizumab in combination with paclitaxel, with or without bevacizumab, was evaluated in KEYNOTE-B96 (NCT05116189), a multicenter, randomized, double-blind, placebo-controlled trial that enrolled 643 patients with platinum-resistant, epithelial ovarian, fallopian tube, or primary peritoneal carcinoma who received one or two prior lines of systemic therapy for ovarian carcinoma. Patients must have received at least one line of platinum-based chemotherapy for ovarian cancer with radiographic evidence of disease progression within 6 months after the last dose. Prior therapy with an anti-PD-1/PD-L1 inhibitor, PARP inhibitor, or bevacizumab was permitted. Patients were enrolled regardless of PD-L1 tumor expression status. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomization was stratified by investigator decision to use bevacizumab, geographic region (U.S. or European Union or Rest of World), and PD-L1 status according to the PD-L1 IHC 22C3 pharmDx assay (CPS <1 or CPS 1 to <10 or CPS ≥10). Patients were randomized (1:1) to one of the two treatment groups:
- Intravenous pembrolizumab 400 mg every 6 weeks plus paclitaxel 80 mg/m2 with or without bevacizumab 10 mg/kg
- Placebo every 6 weeks plus paclitaxel 80 mg/m2 with or without bevacizumab 10 mg/kg
All study medications were administered as an intravenous infusion. Intravenous pembrolizumab 400 mg or placebo were administered on Day 1 of each 6-week treatment cycle and paclitaxel 80 mg/m2 was administered on Days 1, 8, and 15 of each 3-week treatment cycle. The option to use bevacizumab was by investigator choice prior to randomization. Bevacizumab 10 mg/kg was administered on Day 1 of a 2-week treatment cycle. Treatment with intravenous pembrolizumab continued until RECIST v1.1-defined progression of disease, unacceptable toxicity, or a maximum of 24 months. Administration of intravenous pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed every 9 weeks for the first year, followed by every 12 weeks thereafter. The major efficacy outcome measure was PFS as assessed by investigator according to RECIST v1.1. An additional efficacy outcome measure was OS.
Among the 643 patients randomized, 466 patients (72%) had tumors expressing PD-L1 with a CPS ≥1. The population characteristics of these 466 patients were: median age of 62 years (range: 37 to 85), 38% age 65 or older; 67% White, 20% Asian, 8% Missing, 3% Multiple, 2% Black, 0.4% Native Hawaiian or other Pacific Islander; 13% Hispanic or Latino; 55% and 44% ECOG performance status of 0 or 1, respectively; 73% received bevacizumab as study treatment; 36% of patients had received one prior line and 64% had received two prior lines of therapy; prior systemic therapy included: 46% with bevacizumab; 39% with a PARP inhibitor, or 3% with an anti-PD-1/PD-L1 inhibitor. The platinum-free interval following the most recent line of therapy was <3 months in 47% of patients, and 3 to 6 months in 53% of patients.
The study demonstrated statistically significant improvement in PFS and OS for patients randomized to intravenous pembrolizumab in combination with paclitaxel with or without bevacizumab compared to placebo in combination with paclitaxel with or without bevacizumab in patients whose tumors expressed PD-L1 CPS ≥1. Efficacy results are summarized in Table 104 and Figures 46 and 47.
Table 104: Efficacy Results in KEYNOTE-B96 (CPS ≥1) Endpoint Intravenous pembrolizumab
400 mg every 6 weeks
plus paclitaxel with or without
bevacizumab
n=234Placebo
plus paclitaxel with or without
bevacizumab
n=232- * Based on stratified log-rank test (p-Value [one-sided] is compared to an alpha boundary of 0.0116)
- † Based on stratified log-rank test (p-Value [one-sided] is compared to an alpha boundary of 0.0083)
PFS Number of patients with event (%) 162 (69) 180 (78) Median in months (95% CI) 8.3 (7.0, 9.4) 7.2 (6.2, 8.1) Hazard ratio (95% CI) 0.72 (0.58, 0.89) p-Value 0.0014* OS Number of patients with event (%) 157 (67) 175 (75) Median in months (95% CI) 18.2 (15.3, 21.0) 14.0 (12.5, 16.1) Hazard ratio (95% CI) 0.76 (0.61, 0.94) p-Value 0.0053† Figure 46: Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-B96 (CPS ≥1) 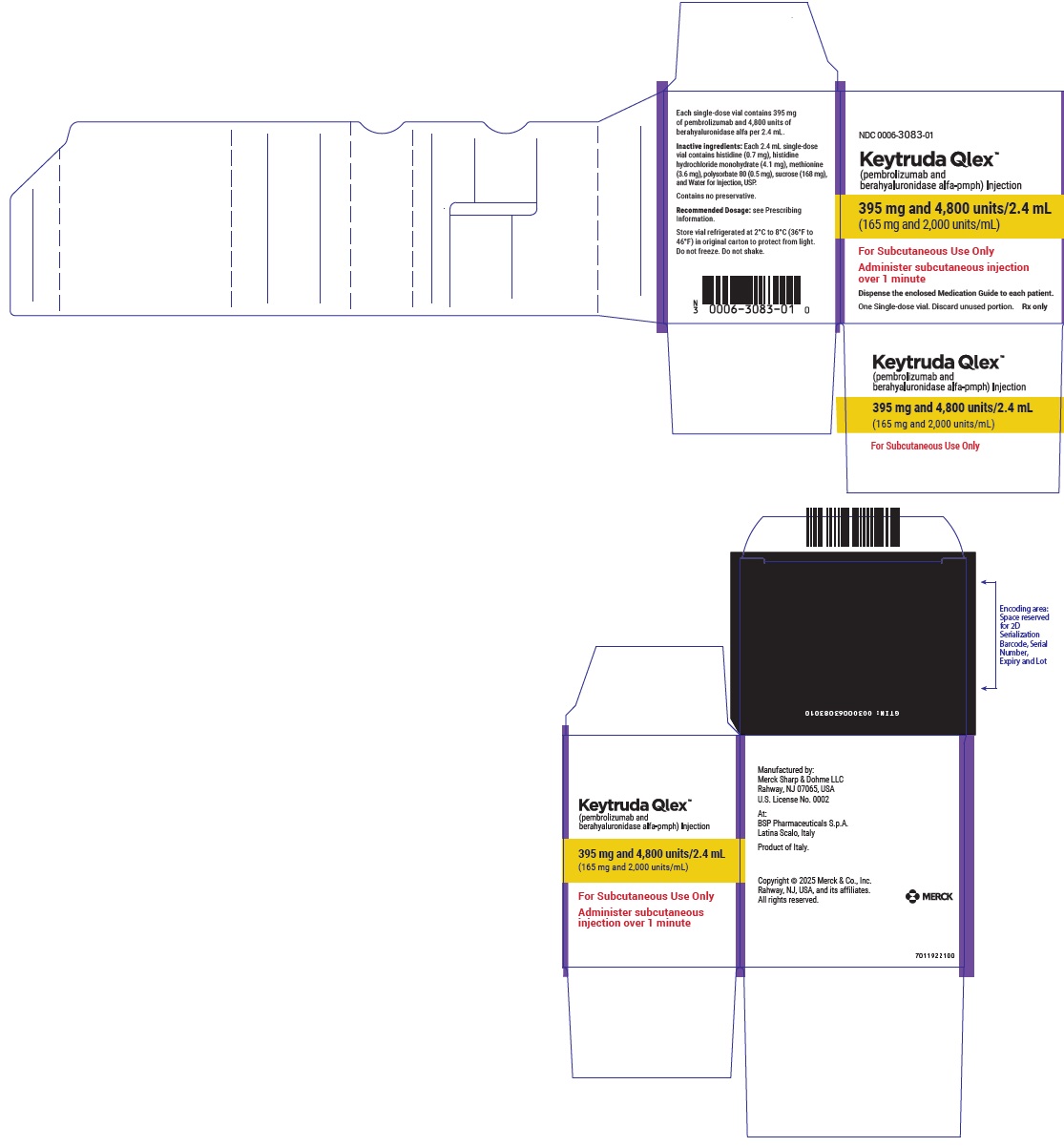
Figure 47: Kaplan-Meier Curve for Overall Survival in KEYNOTE-B96 (CPS ≥1) 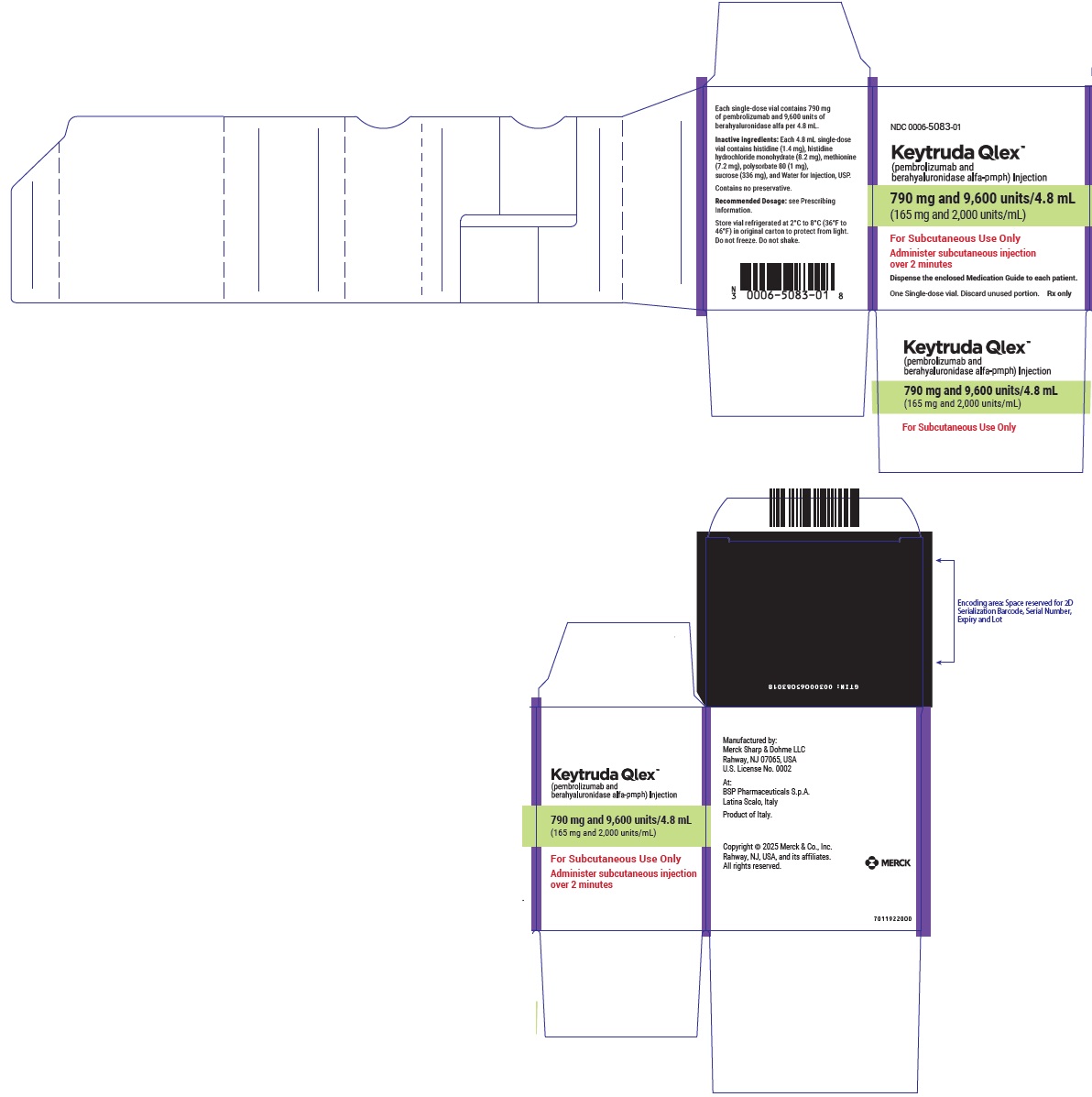
-
16 HOW SUPPLIED/STORAGE AND HANDLING
KEYTRUDA QLEX (pembrolizumab and berahyaluronidase alfa-pmph) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution supplied in single-dose vials for subcutaneous administration. Each carton contains one single-dose vial either as:
- 395 mg pembrolizumab and 4,800 units berahyaluronidase alfa per 2.4 mL (165 mg/ 2,000 units per mL), NDC: 0006-3083-01
- 790 mg pembrolizumab and 9,600 units berahyaluronidase alfa per 4.8 mL (165 mg/ 2,000 units per mL), NDC: 0006-5083-01
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-authorized patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
- Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of KEYTRUDA QLEX. These reactions may include:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for new or worsening cough, chest pain, or shortness of breath [see Warnings and Precautions (5.1)].
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea or severe abdominal pain [see Warnings and Precautions (5.1)].
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, or easy bruising or bleeding [see Warnings and Precautions (5.1)].
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, or Type 1 diabetes mellitus [see Warnings and Precautions (5.1)].
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis [see Warnings and Precautions (5.1)].
- Severe skin reactions: Advise patients to contact their healthcare provider immediately for any signs or symptoms of severe skin reactions, SJS or TEN [see Warnings and Precautions (5.1)].
- Other immune-mediated adverse reactions:
- Advise patients that immune-mediated adverse reactions can occur and may involve any organ system, and to contact their healthcare provider immediately for any new or worsening signs or symptoms [see Warnings and Precautions (5.1)].
- Advise patients of the risk of solid organ transplant rejection and other transplant (including corneal graft) rejection. Advise patients to contact their healthcare provider immediately for signs or symptoms of organ transplant rejection and other transplant (including corneal graft) rejection [see Warnings and Precautions (5.1)].
Hypersensitivity and Administration-Related Reactions
- Advise patients to contact their healthcare provider immediately for signs or symptoms of hypersensitivity and administration-related systemic reactions [see Warnings and Precautions (5.2)].
Complications of Allogeneic HSCT
- Advise patients of the risk of post-allogeneic hematopoietic stem cell transplantation complications [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5), Use in Specific Populations (8.1, 8.3)].
- Advise females of reproductive potential to use effective contraception during treatment with KEYTRUDA QLEX and for 4 months after the last dose [see Warnings and Precautions (5.5), Use in Specific Populations (8.1, 8.3)].
Lactation
- Advise women not to breastfeed during treatment with KEYTRUDA QLEX and for 4 months after the last dose [see Use in Specific Populations (8.2)].
Laboratory Tests
- Advise patients of the importance of keeping scheduled appointments for blood work or other laboratory tests [see Warnings and Precautions (5.1)].
- Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of KEYTRUDA QLEX. These reactions may include:
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USA
U.S. License No. 0002For patent information: www.msd.com/research/patent
The trademarks depicted herein are owned by their respective owners.
Copyright © 2026 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-mk3475a-i-2602r003
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 02/2026 MEDICATION GUIDE
KEYTRUDA QLEX™ (key-true-duh Q-lex)
(pembrolizumab and berahyaluronidase alfa-pmph)
injection, for subcutaneous useWhat is the most important information I should know about KEYTRUDA QLEX?
KEYTRUDA QLEX is a medicine that may treat certain cancers by working with your immune system. KEYTRUDA QLEX can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended. Call or see your healthcare provider right away if you develop any new or worsening signs or symptoms, including: Lung problems - cough
- shortness of breath
- chest pain
Intestinal problems - diarrhea (loose stools) or more frequent bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
Liver problems - yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems - headaches that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems - decrease in your amount of urine
- blood in your urine
- swelling of your ankles
- loss of appetite
Skin problems - rash
- itching
- skin blistering or peeling
- painful sores or ulcers in your mouth or in your nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with KEYTRUDA QLEX. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include: - chest pain, irregular heartbeat, shortness of breath, swelling of ankles
- confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- double vision, blurry vision, sensitivity to light, eye pain, changes in eyesight
- persistent or severe muscle pain or weakness, muscle cramps
- low red blood cells, bruising
Allergic and injection-related reactions that can sometimes be severe or life-threatening, can happen during treatment with KEYTRUDA QLEX. Tell your healthcare provider right away if you get any signs or symptoms, including: - chills or shaking
- itching or rash
- flushing
- shortness of breath or wheezing
- dizziness
- feeling like passing out
- fever
- back pain
Rejection of a transplanted organ or tissue. Your healthcare provider should tell you what signs and symptoms you should report and monitor you depending on the type of organ or tissue transplant that you have had. Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with KEYTRUDA QLEX. Your healthcare provider will monitor you for these complications. Getting medical treatment right away may help keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during treatment with KEYTRUDA QLEX. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with KEYTRUDA QLEX if you have severe side effects.
What is KEYTRUDA QLEX? KEYTRUDA QLEX is a prescription medicine used to treat: - a kind of skin cancer called melanoma. KEYTRUDA QLEX may be used:
- when your melanoma has spread or cannot be removed by surgery (advanced melanoma), or
- in adults and children 12 years of age and older with Stage IIB, Stage IIC, or Stage III melanoma, to help prevent melanoma from coming back after it and lymph nodes that contain cancer have been removed by surgery.
- a kind of lung cancer called non-small cell lung cancer (NSCLC).
- KEYTRUDA QLEX may be used with the chemotherapy medicines pemetrexed and a platinum as your first treatment when your lung cancer:
- has spread (advanced NSCLC), and
- is a type called “nonsquamous”, and
- your tumor does not have an abnormal “EGFR” or “ALK” gene.
- KEYTRUDA QLEX may be used with the chemotherapy medicines carboplatin and either paclitaxel or paclitaxel protein-bound as your first treatment when your lung cancer:
- has spread (advanced NSCLC), and
- is a type called “squamous”.
- KEYTRUDA QLEX may be used alone as your first treatment when your lung cancer:
- has not spread outside your chest (Stage III) and you cannot have surgery or chemotherapy with radiation or
- your NSCLC has spread to other areas of your body (advanced NSCLC), and
- your tumor tests positive for “PD-L1”, and
- does not have an abnormal “EGFR” or “ALK” gene.
- KEYTRUDA QLEX may also be used alone when:
- you have received chemotherapy that contains platinum to treat your advanced NSCLC, and it did not work or it is no longer working, and
- your tumor tests positive for “PD-L1”, and
- if your tumor has an abnormal “EGFR” or “ALK” gene, you have also received an EGFR or ALK inhibitor medicine and it did not work or is no longer working.
- KEYTRUDA QLEX may be used in combination with chemotherapy that contains platinum and another chemotherapy medicine:
- before surgery when you have early-stage NSCLC which can be removed by surgery, and
- then continued alone after surgery to help prevent your lung cancer from coming back.
- KEYTRUDA QLEX may be used alone as a treatment in adults for your lung cancer:
- to help prevent your lung cancer from coming back after your tumor(s) has been removed by surgery and you have received platinum-based chemotherapy, and
- you have Stage IB and your tumor(s) is 4 cm or greater in size, Stage II, or Stage IIIA NSCLC.
- KEYTRUDA QLEX may be used with the chemotherapy medicines pemetrexed and a platinum as your first treatment when your lung cancer:
- a kind of cancer in adults called malignant pleural mesothelioma (MPM) that affects the lining of the lungs and chest wall.
- KEYTRUDA QLEX may be used in combination with the chemotherapy medicines pemetrexed and a platinum as your first treatment when your cancer has spread or cannot be removed by surgery (advanced MPM).
- a kind of cancer called head and neck squamous cell cancer (HNSCC).
- KEYTRUDA QLEX may be used alone in adults with HNSCC before surgery:
- when your cancer can be removed by surgery, has spread to nearby tissues, and your tumor tests positive for “PD-L1”, and
- then continued in combination with radiation with or without cisplatin after surgery, and
- then continued alone to help prevent your head and neck cancer from coming back.
- KEYTRUDA QLEX may be used with the chemotherapy medicines fluorouracil and a platinum as your first treatment when your head and neck cancer has spread or returned and cannot be removed by surgery.
- KEYTRUDA QLEX may be used alone as your first treatment when your head and neck cancer:
- has spread or returned and cannot be removed by surgery, and
- your tumor tests positive for “PD-L1”.
- KEYTRUDA QLEX may be used alone when your head and neck cancer:
- has spread or returned, and
- you have received chemotherapy that contains platinum and it did not work or is no longer working.
- KEYTRUDA QLEX may be used alone in adults with HNSCC before surgery:
- a kind of bladder and urinary tract cancer called urothelial cancer.
- KEYTRUDA QLEX may be used with the medicine enfortumab vedotin in adults when your bladder or urinary tract cancer has spread or cannot be removed by surgery (locally advanced or metastatic urothelial cancer).
- KEYTRUDA QLEX may be used alone when your bladder or urinary tract cancer:
- has spread or cannot be removed by surgery (locally advanced or metastatic urothelial cancer), and
- you are not able to receive chemotherapy that contains platinum (medicines called either cisplatin or carboplatin), or
- you have received chemotherapy that contains platinum, and it did not work or is no longer working.
- KEYTRUDA QLEX may be used with the medicine enfortumab vedotin in adults before and after the surgical removal of your bladder when:
- your bladder cancer has spread into the muscle layer of the bladder (muscle invasive bladder cancer [MIBC]) but not to other parts of the body, and
- you are not able to receive chemotherapy that contains cisplatin.
- KEYTRUDA QLEX may be used alone when your cancer has not spread to nearby tissue in the bladder, but is at high-risk for spreading (high-risk non-muscle-invasive bladder cancer [NMIBC]) when:
- your tumor is a type called “carcinoma in situ” (CIS), and
- you have tried treatment with Bacillus Calmette-Guerin (BCG) and it did not work, and
- you are not able to or have decided not to have surgery to remove your bladder.
- a kind of cancer that is shown by a laboratory test to be a microsatellite instability-high (MSI-H) or a mismatch repair deficient (dMMR) solid tumor. KEYTRUDA QLEX may be used in adults and children 12 years of age and older to treat:
- cancer that has spread or cannot be removed by surgery (advanced cancer), and
- has progressed following treatment, and you have no satisfactory treatment options.
- a kind of cancer called colon or rectal cancer. KEYTRUDA QLEX may be used when your cancer:
- has spread or cannot be removed by surgery (advanced colon or rectal cancer), and
- has been shown by a laboratory test to be microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR).
- a kind of stomach cancer called gastric or gastroesophageal junction (GEJ) adenocarcinoma.
- KEYTRUDA QLEX may be used in adults in combination with the medicine trastuzumab along with fluoropyrimidine and platinum chemotherapy as your first treatment when your stomach cancer:
- is HER2-positive, and your tumor tests positive for “PD-L1”, and
- has spread or cannot be removed by surgery (advanced gastric cancer).
- KEYTRUDA QLEX may be used in adults in combination with fluoropyrimidine and platinum chemotherapy as your first treatment when your stomach cancer:
- is HER2-negative, and your tumor tests positive for “PD-L1”, and
- has spread or cannot be removed by surgery (advanced gastric cancer).
- KEYTRUDA QLEX may be used in adults in combination with the medicine trastuzumab along with fluoropyrimidine and platinum chemotherapy as your first treatment when your stomach cancer:
- a kind of cancer called esophageal or certain gastroesophageal junction (GEJ) carcinomas that cannot be cured by surgery or a combination of chemotherapy and radiation therapy.
- KEYTRUDA QLEX may be used in combination with platinum- and fluoropyrimidine-based chemotherapy medicines when your tumor tests positive for “PD-L1”.
- KEYTRUDA QLEX may be used alone when:
- you have received one or more types of treatment, and it did not work or it is no longer working, and
- your tumor is a type called “squamous”, and
- your tumor tests positive for “PD-L1”.
- a kind of cancer called cervical cancer.
- KEYTRUDA QLEX may be used with chemotherapy and radiation therapy when your cervical cancer has spread nearby to the lower part of your vagina or to pelvic organs or has affected your kidneys (Stage III to IVA FIGO 2014 classification).
- KEYTRUDA QLEX may be used with chemotherapy medicines, with or without the medicine bevacizumab, when:
- your cervical cancer does not go away (persistent), has returned, or has spread (advanced cervical cancer), and
- your tumor tests positive for “PD-L1”.
- KEYTRUDA QLEX may be used alone when your cervical cancer:
- has returned, or has spread (advanced cervical cancer), and
- you have received chemotherapy, and it did not work or is no longer working, and
- your tumor tests positive for “PD-L1”.
- a kind of liver cancer called hepatocellular carcinoma (HCC). KEYTRUDA QLEX may be used when:
- you have HCC after having hepatitis B, and
- you have received anti-cancer treatment that did not contain a “PD-1” or “PD-L1” blocking medicine.
- a kind of bile duct or gallbladder cancer called biliary tract cancer (BTC). KEYTRUDA QLEX may be used with chemotherapy medicines gemcitabine and cisplatin when your biliary tract cancer has spread or cannot be removed by surgery.
- a kind of skin cancer called Merkel cell carcinoma (MCC) in adults and children 12 years of age and older. KEYTRUDA QLEX may be used to treat your skin cancer when it has spread or returned.
- a kind of kidney cancer called renal cell carcinoma (RCC).
- KEYTRUDA QLEX may be used in adults with the medicine axitinib as your first treatment when your kidney cancer has spread or cannot be removed by surgery (advanced RCC).
- KEYTRUDA QLEX may be used in adults with the medicine lenvatinib as your first treatment when your kidney cancer has spread or cannot be removed by surgery (advanced RCC).
- KEYTRUDA QLEX may be used alone if you are at intermediate-high or high risk of your kidney cancer (RCC) coming back after surgery to:
- remove all or part of your kidney, or
- remove all or part of your kidney and also surgery to remove cancer that has spread to other parts of the body (metastatic lesions).
- a kind of uterine cancer called advanced endometrial carcinoma.
- KEYTRUDA QLEX may be used with the chemotherapy medicines carboplatin and paclitaxel, and then KEYTRUDA QLEX may be used alone, in adults:
- when your cancer has spread (advanced), or
- if your cancer has returned.
- KEYTRUDA QLEX may be used with the medicine lenvatinib in adults:
- when a laboratory test shows that your tumor is mismatch repair proficient (pMMR) or not microsatellite instability-high (MSI-H), and
- you have received anti-cancer treatment, and it is no longer working, and
- your cancer cannot be cured by surgery or radiation.
- KEYTRUDA QLEX may be used alone in adults:
- if your cancer is shown by a laboratory test to be microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), and
- you have received anti-cancer treatment and it is no longer working, and
- your cancer cannot be cured by surgery or radiation.
- KEYTRUDA QLEX may be used with the chemotherapy medicines carboplatin and paclitaxel, and then KEYTRUDA QLEX may be used alone, in adults:
- a kind of cancer that is shown by a test to be tumor mutational burden-high (TMB-H). KEYTRUDA QLEX may be used in adults and children 12 years of age and older to treat:
- solid tumors that have spread or cannot be removed by surgery (advanced cancer), and
- you have received anti-cancer treatment, and it did not work or is no longer working, and
- you have no satisfactory treatment options.
- a kind of skin cancer called cutaneous squamous cell carcinoma (cSCC). KEYTRUDA QLEX may be used when your skin cancer:
- has returned or spread, and
- cannot be cured by surgery or radiation.
- a kind of cancer called triple-negative breast cancer (TNBC).
- KEYTRUDA QLEX may be used with chemotherapy medicines as treatment before surgery and then continued alone after surgery when you:
- have early-stage breast cancer, and
- are at high risk of your breast cancer coming back.
- KEYTRUDA QLEX may be used with chemotherapy medicines when your breast cancer:
- has returned and cannot be removed by surgery or has spread, and
- tests positive for “PD-L1”.
- KEYTRUDA QLEX may be used with chemotherapy medicines as treatment before surgery and then continued alone after surgery when you:
- a kind of cancer called ovarian cancer.
- KEYTRUDA QLEX may be used in adults with the chemotherapy medicine paclitaxel, with or without the medicine bevacizumab, when your ovarian, fallopian tube, or primary peritoneal cancer:
- is resistant to chemotherapy that contains platinum, and
- tests positive for “PD-L1”, and
- you have received 1or 2 types of treatment.
- KEYTRUDA QLEX may be used in adults with the chemotherapy medicine paclitaxel, with or without the medicine bevacizumab, when your ovarian, fallopian tube, or primary peritoneal cancer:
Who should not receive KEYTRUDA QLEX?
Do not receive KEYTRUDA QLEX if you are allergic to berahyaluronidase, hyaluronidase or any of the inactive ingredients in KEYTRUDA QLEX. See the end of this Medication Guide for a complete list of ingredients in KEYTRUDA QLEX.Before receiving KEYTRUDA QLEX, tell your healthcare provider about all of your medical conditions, including if you: - have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus
- have received an organ or tissue transplant, including corneal transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have received radiation treatment to your chest area
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- are pregnant or plan to become pregnant. KEYTRUDA QLEX can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider will give you a pregnancy test before you start treatment with KEYTRUDA QLEX.
- You should use an effective method of birth control during treatment with KEYTRUDA QLEX and for 4 months after the last dose of KEYTRUDA QLEX. Talk to your healthcare provider about birth control methods that you can use during this time.
- Tell your healthcare provider right away if you think you may be pregnant or if you become pregnant during treatment with KEYTRUDA QLEX.
- are breastfeeding or plan to breastfeed. It is not known if KEYTRUDA QLEX passes into your breast milk. Do not breastfeed during treatment with KEYTRUDA QLEX and for 4 months after your last dose of KEYTRUDA QLEX.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. How will I receive KEYTRUDA QLEX? - Your healthcare provider will give you KEYTRUDA QLEX as an injection under the skin (subcutaneous) in the stomach area (abdomen) or thigh.
- KEYTRUDA QLEX is usually given every 3 weeks over 1 minute or every 6 weeks over 2 minutes depending on the dose of KEYTRUDA QLEX that you are receiving.
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider will do blood tests to check you for side effects.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of KEYTRUDA QLEX? KEYTRUDA QLEX can cause serious side effects. See “What is the most important information I should know about KEYTRUDA QLEX?” The most common side effects of KEYTRUDA QLEX when given with certain chemotherapy medicines include: nausea, tiredness, and muscle, bone, and joint pain. The most common side effects seen with pembrolizumab given into the vein (intravenous pembrolizumab), which may happen with KEYTRUDA QLEX, are shown below: - when used alone include: feeling tired, pain, including pain in muscles, rash, diarrhea, fever, cough, decreased appetite, itching, shortness of breath, constipation, bones or joints and stomach-area (abdominal) pain, nausea, and low levels of thyroid hormone.
- when used alone that are more common in children than in adults include: fever, vomiting, headache, stomach area (abdominal) pain, and low levels of white blood cells.
- when used with certain chemotherapy or chemotherapy with radiation therapy medicines include: feeling tired or weak, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, trouble breathing, fever, hair loss, inflammation of the nerves that may cause pain, weakness, and paralysis in the arms and legs, swelling of the lining of the mouth, nose, eyes, throat, intestines, or vagina, mouth sores, headache, weight loss, stomach-area (abdominal) pain, joint and muscle pain, trouble sleeping, blisters or rash on the palms of your hands and soles of your feet, urinary tract infection, low levels of thyroid hormone, skin irritation in the radiation area, trouble swallowing, and dry mouth.
- when used with chemotherapy and bevacizumab include: tingling or numbness of the arms or legs, hair loss, low red blood cell count, feeling tired or weak, nausea, low white blood cell count, diarrhea, high blood pressure, decreased platelet count, constipation, joint aches, vomiting, urinary tract infection, rash, low levels of thyroid hormone, decreased appetite, fever, mouth sores, and nosebleed.
- when used with axitinib include: diarrhea, feeling tired or weak, high blood pressure, liver problems, low levels of thyroid hormone, decreased appetite, blisters or rash on the palms of your hands and soles of your feet, nausea, mouth sores or swelling of the lining of the mouth, nose, eyes, throat, intestines, or vagina, hoarseness, rash, cough, and constipation.
- when used with lenvatinib include: low levels of thyroid hormone, high blood pressure, feeling tired, diarrhea, joint and muscle pain, nausea, decreased appetite, vomiting, mouth sores, weight loss, stomach-area (abdominal) pain, urinary tract infection, protein in your urine, constipation, headache, bleeding, blisters or rash on the palms of your hands and soles of your feet, hoarseness, rash, liver problems, and kidney problems.
- when used with enfortumab vedotin include: rash, tingling or numbness of the arms or legs, feeling tired, itching, diarrhea, hair loss, weight loss, decreased appetite, dry eye, nausea, constipation, changes in sense of taste, and urinary tract infection.
These are not all of the possible side effects of KEYTRUDA QLEX.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of KEYTRUDA QLEX Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about KEYTRUDA QLEX that is written for health professionals. What are the ingredients in KEYTRUDA QLEX? Active ingredients: pembrolizumab and berahyaluronidase alfa-pmph Inactive ingredients: histidine, histidine hydrochloride monohydrate, methionine, polysorbate 80, sucrose, and Water for Injection. Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USA
U.S. License No. 0002For patent information: www.msd.com/research/patent
Copyright © 2026 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.
usmg-mk3475a-i-2602r003
For more information, go to www.keytruda.com/qlex. -
PRINCIPAL DISPLAY PANEL - 395 mg and 4,800 units/2.4 mL Vial Carton
NDC: 0006-3083-01
Keytruda Qlex ™
(pembrolizumab and
berahyaluronidase alfa-pmph) Injection395 mg and 4,800 units/2.4 mL
(165 mg and 2,000 units/mL)For Subcutaneous Use Only
Administer subcutaneous injection
over 1 minuteDispense the enclosed Medication Guide to each patient.
One Single-dose vial. Discard unused portion.
Rx only
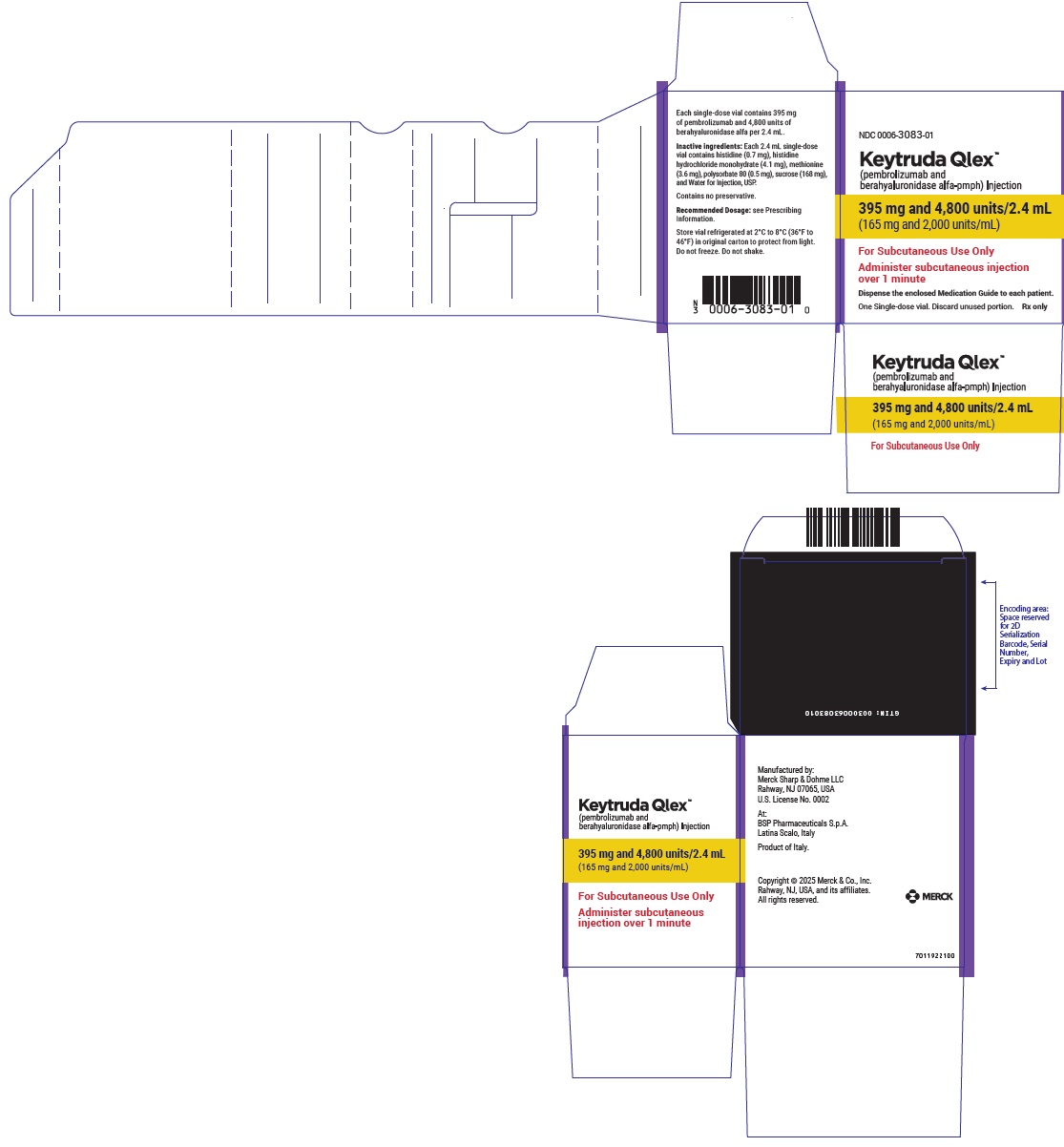
-
PRINCIPAL DISPLAY PANEL - 790 mg and 9,600 units/4.8 mL Vial Carton
NDC: 0006-5083-01
Keytruda Qlex ™
(pembrolizumab and
berahyaluronidase alfa-pmph) Injection790 mg and 9,600 units/4.8 mL
(165 mg and 2,000 units/mL)For Subcutaneous Use Only
Administer subcutaneous injection
over 2 minutesDispense the enclosed Medication Guide to each patient.
One Single-dose vial. Discard unused portion.
Rx only
-
INGREDIENTS AND APPEARANCE
KEYTRUDA QLEX
pembrolizumab and berahyaluronidase alfa-pmph injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-5083 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEMBROLIZUMAB (UNII: DPT0O3T46P) (PEMBROLIZUMAB - UNII:DPT0O3T46P) PEMBROLIZUMAB 165 mg in 1 mL BERAHYALURONIDASE ALFA (UNII: 9X4R4A4HQQ) (BERAHYALURONIDASE ALFA - UNII:9X4R4A4HQQ) BERAHYALURONIDASE ALFA 2000 U in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 0.3 mg in 1 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 1.7 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 70 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-5083-01 1 in 1 CARTON 09/19/2025 1 NDC: 0006-5083-99 4.8 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761467 09/19/2025 KEYTRUDA QLEX
pembrolizumab and berahyaluronidase alfa-pmph injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-3083 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEMBROLIZUMAB (UNII: DPT0O3T46P) (PEMBROLIZUMAB - UNII:DPT0O3T46P) PEMBROLIZUMAB 165 mg in 1 mL BERAHYALURONIDASE ALFA (UNII: 9X4R4A4HQQ) (BERAHYALURONIDASE ALFA - UNII:9X4R4A4HQQ) BERAHYALURONIDASE ALFA 2000 U in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 0.3 mg in 1 mL HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) 1.7 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 70 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-3083-01 1 in 1 CARTON 09/19/2025 1 NDC: 0006-3083-99 2.4 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761467 09/19/2025 Labeler - Merck Sharp & Dohme LLC (118446553)
Trademark Results [KEYTRUDA QLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KEYTRUDA QLEX 98256332 not registered Live/Pending |
MERCK SHARP & DOHME LLC 2023-11-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.