ALFENTANIL HYDROCHLORIDE injection
Alfentanil Hydrochloride by
Drug Labeling and Warnings
Alfentanil Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Akorn, Akorn Operating Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALFENTANIL HCl INJECTION safely and effectively. See full prescribing information for ALFENTANIL HCl INJECTION.
ALFENTANIL HCl Injection, for Intravenous use, CII
Initial U.S. Approval: 1986WARNING: ADDICTION, ABUSE, AND MISUSE
See full prescribing information for complete boxed warning.
- Alfentanil HCl injection exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient's risk before prescribing and monitor closely for these behaviors and conditions. (5.1)
RECENT MAJOR CHANGES
Warnings and Precautions (5.2) 10/2019 INDICATIONS AND USAGE
Alfentanil HCl Injection is an opioid indicated:
- as an analgesic adjunct given in incremental doses in the maintenance of anesthesia with barbiturate/nitrous oxide/oxygen.
- as an analgesic administered by continuous infusion with nitrous oxide/oxygen in the maintenance of general anesthesia
- as a primary anesthetic agent for the induction of anesthesia in patients undergoing general surgery in which endotracheal intubation and mechanical ventilation are required.
- as the analgesic component for monitored anesthesia care (MAC) (1)
DOSAGE AND ADMINISTRATION
- Alfentanil HCl Injection should be administered only by persons specifically trained in the use of intravenous anesthetics and management of the respiratory effects of potent opioids.
- Ensure that an opioid antagonist, resuscitative and intubation equipment, and oxygen are readily available (2.1).
- Individualize dosing based on factors such as age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used, and the surgical procedure involved. (2.1)
- Doses of Alfentanil HCl Injection vary depending on circumstances (see dosing chart, 2.2.)
- Reduce the dose of Alfentanil HCl Injection in elderly, debilitated and obese patients. (2.4, 2.5)
- Reduce the dose of Alfentanil HCl Injection when administered with CNS depressants. (2.6)
DOSAGE FORMS AND STRENGTHS
Solution for injection (sterile): eq. to 500 mcg/mL alfentanil base; 2 mL, 5 mL, 10 mL and 20 mL ampules. (3)
CONTRAINDICATIONS
- Hypersensitivity to alfentanil (4)
WARNINGS AND PRECAUTIONS
- Risks of Muscle Rigidity and Skeletal Muscle Movement: Manage with neuromuscular blocking agent. See full prescribing information for more detail on managing these risks. (5.4)
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, and Debilitated Patients: Monitor closely, particularly during initiation and titration. (5.2)
- Serotonin Syndrome: Potentially life-threatening condition could result from concomitant serotonergic drug administration. Discontinue Alfentanil HCl Injection if serotonin syndrome is suspected. (5.6)
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, and Head Injury: Monitor for sedation and respiratory depression. (5.9)
ADVERSE REACTIONS
Most common adverse reactions were apnea, rigidity, and bradycardia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Akorn, Inc. at 1-800-932-5676 or FDA at 1-800-FDA-1088 or www.fda.gov.medwatch.
DRUG INTERACTIONS
- Concomitant Use of CNS Depressants: May decrease pulmonary arterial pressure and may cause hypotension. See FPI for management instructions. For post-operative pain, start with the lowest effective dosage and monitor for potentiation of CNS depressant effects. (5.5, 7)
- Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with Alfentanil HCl Injection because they may reduce analgesic effect of Alfentanil HCl Injection or precipitate withdrawal symptoms. (7)
USE IN SPECIFIC POPULATIONS
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ADDICTION, ABUSE, AND MISUSE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Dosage
2.3 Discontinuation of Alfentanil HCl Injection
2.4 Dosage Modification in Elderly Patients
2.5 Dosage Modifications in Obese Patients
2.6 Dosage Modifications with Concomitant Use with Other CNS Depressants
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Addiction, Abuse, and Misuse
5.2 Life-Threatening Respiratory Depression
5.3 Risks of Concomitant Use or Discontinuation of Cytochrome P450 3A4 Inhibitors and Inducers
5.4 Risks of Muscle Rigidity and Skeletal Muscle Movement
5.5 Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
5.6 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
5.7 Potentiation of Monoamine Oxidase Inhibitors
5.8 Bradycardia
5.9 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, or Head Injury
5.10 Risks of Use in Patients with Gastrointestinal Conditions
5.11 Increased Risk of Seizures in Patients with Convulsive or Seizure Disorders
5.12 Risks due to Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Respiratory Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ADDICTION, ABUSE, AND MISUSE
Addiction, Abuse, and Misuse
Alfentanil HCl injection exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient's risk prior to prescribing Alfentanil HCl Injection, and monitor all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
Alfentanil HCl Injection is indicated:
- as an analgesic adjunct given in incremental doses in the maintenance of anesthesia with barbiturate/nitrous oxide/oxygen.
- as an analgesic administered by continuous infusion with nitrous oxide/oxygen in the maintenance of general anesthesia.
- as a primary anesthetic agent for the induction of anesthesia in patients undergoing general surgery in which endotracheal intubation and mechanical ventilation are required.
- as the analgesic component for monitored anesthesia care (MAC).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Alfentanil HCl Injection should be administered only by persons specifically trained in the use of intravenous anesthetics and management of the respiratory effects of potent opioids.
In patients administered high doses of Alfentanil HCl Injection, it is essential that qualified personnel and adequate facilities are available for the management of postoperative respiratory depression.
- Ensure that an opioid antagonist, resuscitative and intubation equipment, and oxygen are readily available.
- Individualize dosage based on factors such as age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used, and the surgical procedure involved.
- The selection of preanesthetic medications should be based upon the needs of the individual patient.
- The neuromuscular blocking agent selected should be compatible with the patient's condition, taking into account the hemodynamic effects of a particular muscle relaxant and the degree of skeletal muscle relaxation required.
- Patients receiving monitored anesthesia care (MAC) should be continuously monitored by persons not involved in the conduct of the surgical or diagnostic procedure; oxygen supplementation should be immediately available and provided where clinically indicated; oxygen saturation should be continuously monitored; the patient should be observed for early signs of hypotension, apnea, upper airway obstruction and/or oxygen desaturation.
- Delayed respiratory depression, respiratory arrest, bradycardia, asystole, arrhythmias and hypotension have also been reported. Therefore, vital signs must be monitored continuously, including following the termination of surgery.
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- For purposes of administering small volumes of Alfentanil HCl Injection accurately, the use of a tuberculin syringe or equivalent is recommended.
As with other potent opioids, the respiratory depressant effect of alfentanil may persist longer than the measured analgesic effect. The total dose of all opioid agonists administered should be considered by the practitioner before ordering opioid analgesics during recovery from anesthesia.
If Alfentanil HCl Injection is administered with a CNS depressant, become familiar with the properties of each drug, particularly each product's duration of action. In addition, when such a combination is used, fluids and other countermeasures to manage hypotension should be available [see Warnings and Precautions (5.5)].
The physical and chemical compatibility of Alfentanil HCl Injection have been demonstrated in solution with normal saline, 5% dextrose in normal saline, 5% dextrose in water and Lactated Ringers. Clinical studies of Alfentanil HCl Injection infusion have been conducted with Alfentanil HCl Injection diluted to a concentration range of 25 mcg/mL to 80 mcg/mL.
As an example of the preparation of Alfentanil HCl Injection for infusion, 20 mL of Alfentanil HCl injection added to 230 mL of diluent provides 40 mcg/mL solution of Alfentanil.
2.2 Dosage
The dosage of Alfentanil HCl Injection should be individualized and titrated to the desired effect in each patient according to body weight, physical status, underlying pathological condition, use of other drugs, and type and duration of surgical procedure and anesthesia. The dose of Alfentanil HCl Injection should be reduced in elderly or debilitated patients [see Warnings and Precautions (5.6)].
See Dosing Chart for the use of Alfentanil HCl Injection
- by incremental injection as an analgesic adjunct to anesthesia with barbiturate/nitrous oxide/oxygen for short surgical procedures (expected duration of less than one hour);
- by continuous infusion as a maintenance analgesic with nitrous oxide/oxygen for general surgical procedures; and
- by intravenous injection in anesthetic doses for the induction of anesthesia for general surgical procedures with a minimum expected duration of 45 minutes; and
- by intravenous injection as the analgesic component for monitored anesthesia care (MAC).
When administering Alfentanil as induction doses, administer the dose slowly (over three minutes). Because administration of the induction dose may produce loss of vascular tone and hypotension, consider given to fluid replacement prior to induction.
Table 1: Dosing Chart For Use During General Anesthesia Spontaneously Breathing/Assisted Ventilation Induction of Analgesia: 8 to 20 mcg/kg
Maintenance of Analgesia: 3 to 5 mcg/kg q 5 to 20 min or 0.5 to 1 mcg/kg/min
Total dose: 8 to 40 mcg/kgAssisted or Controlled Ventilation
- Assisted or Controlled Ventilation Incremental Injection
(To attenuate response to laryngoscopy and intubation)Induction of Analgesia: 20 to 50 mcg/kg
Maintenance of Analgesia: 5 to 15 mcg/kg q 5 to 20 min
Total dose: Up to 75 mcg/kgAssisted or Controlled Ventilation
- Continuous Infusion
(To provide attenuation of response to intubation and incision)Induction of Analgesia: 50 to 75 mcg/kg
Maintenance of Analgesia: 0.5 to 3 mcg/kg/min (Average rate 1 to 1.5 mcg/kg/min) Infusion rates are variable and should be titrated to the desired clinical effect. See Infusion Dosage Guidelines Below
Total dose: Dependent on duration of procedureAnesthetic Induction Induction of Anesthesia: 130 to 245 mcg/kg Administer slowly (over 3 minutes).
Maintenance of Anesthesia: 0.5 to 1.5 mcg/kg/min or general anesthetic.
Infusion rates are variable and should be titrated to the desired clinical effect. See Infusion Dosage Guidelines Below
Total dose: Dependent on duration of procedure
At these doses truncal rigidity should be expected and a muscle relaxant should be utilized.
In patients administered anesthetic (induction) dosages of Alfentanil HCl Injection, it is essential that qualified personnel and adequate facilities are available for the management of intraoperative and postoperative respiratory depression.MONITORED ANESTHESIA CARE (MAC)
(For sedated and responsive, spontaneously breathing patients)Induction of MAC: 3 to 8 mcg/kg
Maintenance of MAC: 3 to 5 mcg/kg q 5 to 20 min or 0.25 to 1 mcg/kg/min
Infusion rates are variable and should be titrated to the desired clinical effect. See Infusion Dosage Guidelines Below
Total dose: 3 to 40 mcg/kg
Patients receiving monitored anesthesia care (MAC) should be continuously monitored by persons not involved in the conduct of the surgical or diagnostic procedureInfusion Dosage Guidelines For Continuous Infusion: 0.5 to 3 mcg/kg/min administered with nitrous oxide/oxygen in patients undergoing general surgery.
Following an anesthetic induction dose of Alfentanil HCl Injection, alfentanil infusion rate requirements are reduced by 30 to 50% for the first hour of maintenance. Requirements for volatile inhalation anesthetics are also reduced by 30 to 50% for the first hour of maintenance.
Changes in vital signs that indicate a response to surgical stress or lightening of anesthesia may be controlled by increasing the Alfentanil HCl Injection to a maximum of 4 mcg/kg/min and/or administration of bolus doses of 7 mcg/kg. If changes are not controlled after three bolus doses given over a five-minute period, a barbiturate, vasodilator, and/or inhalation agent should be used. Infusion rates should always be adjusted downward in the absence of these signs until there is some response to surgical stimulation.
Rather than an increase in infusion rate, 7 mcg/kg bolus doses of Alfentanil HCl Injection or a potent inhalation agent should be administered in response to signs of lightening of anesthesia within the last 15 minutes of surgery. Alfentanil HCl Injection infusion should be discontinued at least 10 to 15 minutes prior to the end of surgery.
In patients administered anesthetic (induction) dosages of Alfentanil HCl Injection, it is essential that qualified personnel and adequate facilities are available for the management of intraoperative and postoperative respiratory depression.2.3 Discontinuation of Alfentanil HCl Injection
Alfentanil HCl Injection infusions should be discontinued at least 10 to 15 minutes prior to the end of surgery during general anesthesia. During administration of Alfentanil HCl Injection for Monitored Anesthesia Care (MAC), infusions may be continued to the end of the procedure.
2.4 Dosage Modification in Elderly Patients
Reduce the initial dose of Alfentanil HCl Injection in elderly patients by up to 40% due to reduced clearance and increased sensitivity to the effects. [see Specific Populations (8.5)]. The effect of the initial dose should be considered in determining supplemental doses.
2.5 Dosage Modifications in Obese Patients
In obese patients (more than 20% above ideal body weight) the dose of Alfentanil HCl Injection should be determined on the basis of lean body weight.
2.6 Dosage Modifications with Concomitant Use with Other CNS Depressants
Other CNS depressant drugs (e.g. barbiturates, tranquilizers, narcotics and general anesthetics) will have additive or potentiating effects with Alfentanil HCl Injection. When patients have received such drugs, the dose of Alfentanil HCl Injection, required will be less than usual. Following the administration of Alfentanil HCl Injection, the dose of other CNS depressant drugs should be reduced. [see Drug Interactions (7)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Alfentanil HCl Injection is contraindicated in patients with:
- Hypersensitivity to alfentanil (e.g., anaphylaxis) [see Adverse Reactions (6)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Addiction, Abuse, and Misuse
Alfentanil HCl Injection contains alfentanil, a Schedule II controlled substance. As an opioid, Alfentanil HCl Injection exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence (9)].
Opioids are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. Consider these risks when handling Alfentanil HCl Injection. Strategies to reduce these risks include proper product storage and control practices for a C-II drug.
Contact local and state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
5.2 Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Alfentanil HCl Injection should be administered only by persons specifically trained in the use of anesthetic drugs and the management of the respiratory effects of potent opioids, including respiration and cardiac resuscitation of patients in the age group being treated. Such training must include the establishment and maintenance of a patent airway and assisted ventilation. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered anesthetic doses of Alfentanil HCl Injection. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status [see Overdosage (10)]. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
As with other potent opioids, the respiratory depressant effect of Alfentanil HCl Injection may persist longer than the measured analgesic effect. The total dose of all opioid agonists administered should be considered by the practitioner before ordering opioid analgesics during recovery from anesthesia.
Certain forms of conduction anesthesia, such as spinal anesthesia and some epidural anesthetics, can alter respiration by blocking intercostal nerves [see Clinical Pharmacology (12.2)]. Alfentanil HCl Injection can also alter respiration. Therefore, when Alfentanil HCl Injection is used to supplement these forms of anesthesia, the anesthetist should be familiar with the physiological alterations involved, and be prepared to manage them in the patients selected for these forms of anesthesia.
Patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of Alfentanil HCl Injection. Elderly, cachectic, or debilitated patients may have altered pharmacokinetics or altered clearance compared to younger, healthier patients resulting in greater risk for respiratory depression.
Monitor such patients closely including vital signs, particularly when initiating and titrating Alfentanil HCl Injection and when Alfentanil HCl Injection is given concomitantly with other drugs that depress respiration. To reduce the risk of respiratory depression, proper dosing and titration of Alfentanil HCl Injection are essential [see Dosage and Administration (2)].
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see Dosage and Administration (2.1)].
5.3 Risks of Concomitant Use or Discontinuation of Cytochrome P450 3A4 Inhibitors and Inducers
Concomitant use of Alfentanil HCl Injection with a CYP3A4 inhibitor, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir), may increase plasma concentrations of Alfentanil HCl Injection and prolong opioid adverse reactions, which may exacerbate fatal respiratory depression [see Warnings and Precautions (5.4)], particularly when an inhibitor is added after a stable dose of Alfentanil HCl Injection is achieved. Similarly, discontinuation of a CYP3A4 inducer, such as rifampin, carbamazepine, and phenytoin, in Alfentanil HCl Injection-treated patients may increase alfentanil plasma concentrations and prolong opioid adverse reactions. When using Alfentanil HCl Injection with CYP3A4 inhibitors or discontinuing CYP3A4 inducers in Alfentanil HCl Injection-treated patients, monitor patients closely at frequent intervals and consider dosage reduction of Alfentanil HCl Injection [see Dosage and Administration (2.1), Drug Interactions (7)].
Concomitant use of Alfentanil HCl Injection with CYP3A4 inducers or discontinuation of an CYP3A4 inhibitor could result in lower than expected alfentanil plasma concentrations, and decrease efficacy. When using Alfentanil HCl Injection with CYP3A4 inducers or discontinuing CYP3A4 inhibitors, monitor patients closely at frequent intervals and consider increasing Alfentanil HCl Injection dosage [see Dosage and Administration (2.1), Drug Interactions (7)].
5.4 Risks of Muscle Rigidity and Skeletal Muscle Movement
Alfentanil HCl Injection administered in initial dosages up to 20 mcg/kg may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is usually dose-related. Administration of Alfentanil HCl Injection at anesthetic induction dosages (above 130 mcg/kg) will consistently produce muscular rigidity with an immediate onset. The onset of muscular rigidity occurs earlier than with other opioids. Alfentanil may produce muscular rigidity that involves all skeletal muscles, including those of the neck and extremities. The incidence may be reduced by: 1) routine methods of administration of neuromuscular blocking agents for balanced opioid anesthesia; 2) administration of up to 1/4 of the full paralyzing dose of a neuromuscular blocking agent just prior to administration of Alfentanil HCl Injection at dosages up to 130 mcg/kg; following loss of consciousness, a full paralyzing dose of a neuromuscular blocking agent should be administered; or 3) simultaneous administration of Alfentanil HCl Injection and a full paralyzing dose of a neuromuscular blocking agent when Alfentanil HCl Injection is used in rapidly administered anesthetic dosages (above 130 mcg/kg).
The neuromuscular blocking agent used should be appropriate for the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered Alfentanil HCl Injection. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression.
Patients receiving monitored anesthesia care (MAC) should be continuously monitored by persons not involved in the conduct of the surgical or diagnostic procedure; oxygen supplementation should be immediately available and provided where clinically indicated; oxygen saturation should be continuously monitored; the patient should be observed for early signs of hypotension, apnea, upper airway obstruction and/or oxygen desaturation.
5.5 Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
When Alfentanil HCl Injection is used with CNS depressants, hypotension can occur. If it occurs, consider the possibility of hypovolemia and manage with appropriate parenteral fluid therapy. When operative conditions permit, consider repositioning the patient to improve venous return to the heart. Exercise care in moving and repositioning of patients because of the possibility of orthostatic hypotension. If volume expansion with fluids plus other countermeasures do not correct hypotension, consider administration of pressor agents other than epinephrine. Epinephrine may paradoxically decrease blood pressure in patients treated with a neuroleptic that blocks alpha adrenergic activity [see Drug Interactions (7)].
5.6 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of Alfentanil HCl Injection with serotonergic drugs. Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), and drugs that impair metabolism of serotonin (including MAO inhibitors, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue) [see Drug Interactions (7)]. This may occur within the recommended dosage range.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms generally occurs within several hours to a few days of concomitant use, but may occur later than that. Discontinue Alfentanil HCl Injection if serotonin syndrome is suspected.
5.7 Potentiation of Monoamine Oxidase Inhibitors
Severe and unpredictable potentiation of monoamine oxidase (MAO) inhibitors has been reported rarely with alfentanil. Therefore when Alfentanil HCl Injection is administered to patients who have received MAO inhibitors within 14 days, appropriate monitoring and ready availability of vasodilators and beta-blockers for the treatment of hypertension is recommended.
5.8 Bradycardia
Alfentanil can cause bradycardia. Severe bradycardia and asystole have been successfully treated with atropine and conventional resuscitative methods. Monitor patients closely while receiving Alfentanil HCl Injection had have atropine and other resuscitative equipment present.
5.9 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, or Head Injury
In patients who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), Alfentanil HCl Injection may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of increasing intracranial pressure.
5.10 Risks of Use in Patients with Gastrointestinal Conditions
Alfentanil may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis for worsening symptoms.
5.11 Increased Risk of Seizures in Patients with Convulsive or Seizure Disorders
Alfentanil may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures occurring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during Alfentanil HCl Injection therapy.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described, or described in greater detail, in other sections:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)]
- Life-Threatening Respiratory Depression [see Warnings and Precautions (5.6)]
- Muscle Rigidity and Skeletal Muscle Movement [see Warnings and Precautions (5.4)]
- Interactions with Benzodiazepines and CNS Depressants [see Warnings and Precautions (5.5)]
- Serotonin Syndrome [see Warnings and Precautions (5.7)]
- Bradycardia [see Warnings and Precautions (5.8)]
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.11)]
- Seizures [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reaction information is derived from controlled and open clinical trials in 785 patients who received intravenous Alfentanil HCl Injection during induction and maintenance of general anesthesia. The controlled trials included treatment comparisons with fentanyl, thiopental sodium, enflurane, saline placebo and halothane. The incidence of certain side effects is influenced by the type of use, e.g., chest wall rigidity has a higher reported incidence in clinical trials of Alfentanil HCl Injection induction, and by the type of surgery, e.g., nausea and vomiting have a higher reported incidence in patients undergoing gynecologic surgery. The overall reports of nausea and vomiting with alfentanil were comparable to fentanyl.
Incidence Greater than 1% - Probably Causally Related (Derived from clinical trials)
Gastrointestinal: Nausea (28%), vomiting (18%)
Cardiovascular: Arrhythmia, bradycardia (14%), hypertension (18%), hypotension (10%), tachycardia (12%)
Musculoskeletal: Chest wall rigidity (17%), skeletal muscle movements*
Respiratory: Apnea*, postoperative respiratory depression
Central Nervous System: Blurred vision, dizziness*, sleepiness/postoperative sedation
*Incidence 3% to 9%; all others 1% to 3%
Incidence Less than 1% - Probably Causally Related (Derived from clinical trials)
Adverse events reported in post-marketing surveillance, not seen in clinical trials, are italicized.
Body as a whole: Anaphylaxis
Central Nervous System: Headache*, myoclonic movements, postoperative confusion*, postoperative euphoria*, shivering*
Dermatological: Itching*, urticaria*
Injection Site: Pain*
Musculoskeletal: Skeletal muscle rigidity of neck and extremities
Respiratory: Bronchospasm, hypercarbia*, laryngospasm*
*Incidence 0.3% to 1%
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of alfentanil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
The adverse experience profile from 696 patients receiving alfentanil for Monitored Anesthesia Care (MAC) is similar to the profile established with alfentanil during general anesthesia. Respiratory events reported during MAC included hypoxia, apnea, and bradypnea. Other adverse events reported by patients receiving alfentanil for MAC, in order of decreasing frequency, were nausea, hypotension, vomiting, pruritus, confusion, somnolence and agitation.
-
7 DRUG INTERACTIONS
Table 2 includes clinically significant drug interactions with Alfentanil HCl Injection.
Table 2: Clinically Significant Drug Interactions with Alfentanil HCl Injection Inhibitors of CYP3A4 and CYP2D6 Clinical Impact: The concomitant use of Alfentanil HCl Injection and CYP3A4 inhibitors can increase the plasma concentration of alfentanil, resulting in increased or prolonged opioid effects. These effects could be more pronounced with concomitant use of Alfentanil HCl Injection and CYP2D6 and CYP3A4 inhibitors, particularly when an inhibitor is added after a stable dose of Alfentanil HCl Injection is achieved [see Warnings and Precautions (5.4)].
After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the alfentanil plasma concentration will decrease [see Clinical Pharmacology (12.3)], resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to alfentanil.Intervention: If concomitant use is necessary, consider dosage reduction of Alfentanil HCl Injection until stable drug effects are achieved [see Dosage and Administration (2.2)]. Monitor patients for respiratory depression and sedation at frequent intervals.
If a CYP3A4 inhibitor is discontinued, consider increasing the Alfentanil HCl Injection dosage until stable drug effects are achieved [see Dosage and Administration (2.2)]. Monitor for signs of opioid withdrawal.Examples: Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), protease inhibitors (e.g., ritonavir) CYP3A4 Inducers Clinical Impact: The concomitant use of Alfentanil HCl Injection and CYP3A4 inducers can decrease the plasma concentration of alfentanil [see Clinical Pharmacology (12.3)], resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to alfentanil [see Warnings and Precautions (5.13)].
After stopping a CYP3A4 inducer, as the effects of the inducer decline, the alfentanil plasma concentration will increase [see Clinical Pharmacology (12.3)], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause serious respiratory depression.Intervention: If concomitant use is necessary, consider increasing the Alfentanil HCl Injection dosage until stable drug effects are achieved [see Dosage and Administration (2.2)]. Monitor for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider Alfentanil HCl Injection dosage reduction and monitor for signs of respiratory depression. Examples: Rifampin, carbamazepine, phenytoin Benzodiazepines and Other Central Nervous System (CNS) Depressants Clinical Impact: Diazepam administered immediately prior to or in conjunction with high doses of Alfentanil HCl Injection may produce vasodilation and hypotension, and may result in delayed recovery. Both the magnitude and duration of central nervous system and cardiovascular effects may be enhanced when Alfentanil HCl Injection is administered in combination with other CNS depressants such as barbiturates, tranquilizers, opioids, or inhalation general anesthetics. Postoperative respiratory depression may be enhanced or prolonged by these agents. Intervention: Monitor patients receiving Alfentanil HCl Injection and benzodiazepines or other CNS depressants for hypotension patients and prolonged respiratory depression and sedation. In such cases of combined treatment, the dose of one or both agents should be reduced. Limited clinical experience indicates that requirements for volatile inhalation anesthetics are reduced by 30 to 50% for the first sixty (60) minutes following alfentanil induction. [see Warnings and Precautions (5.2)]. Examples: Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol. Serotonergic Drugs Clinical Impact: The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome [see Warnings and Precautions (5.7)]. Intervention: If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue Alfentanil HCl Injection if serotonin syndrome is suspected. Examples: Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that effect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Monoamine Oxidase Inhibitors Clinical Impact: Severe and unpredictable potentiation of monoamine oxidase (MAO) inhibitors has been reported rarely with alfentanil. MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.2)] Intervention: When Alfentanil HCl Injection is administered to patients who have received MAO inhibitors within 14 days, monitor patients for hypertension and ensure ready availability of vasodilators and beta-blockers for the treatment of hypertension as needed. Examples: phenelzine, tranylcypromine, linezolid Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics Clinical Impact: May reduce the analgesic effect of Alfentanil HCl Injection and/or precipitate withdrawal symptoms. Intervention: Avoid concomitant use. Examples: butorphanol, nalbuphine, pentazocine, buprenorphine Muscle Relaxants Clinical Impact: Alfentanil may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. Intervention: Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of Alfentanil HCl Injection and/or the muscle relaxant as necessary. Diuretics Clinical Impact: Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. Intervention: Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. Anticholinergic Drugs Clinical Impact: The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. Intervention: Monitor patients for signs of urinary retention or reduced gastric motility when Alfentanil HCl Injection is used concomitantly with anticholinergic drugs. Cimetidine Clinical Impact: Cimetidine reduces the clearance of alfentanil, extending the duration of action. Intervention: Use smaller alfentanil doses for prolonged administration and monitor closely for respiratory depression and other effects of alfentanil. Nitrous oxide Clinical Impact: Nitrous oxide has been reported to produce cardiovascular depression when given with higher doses of Alfentanil HCl Injection. Intervention: Monitor patients for signs of cardiovascular depression that may be greater than otherwise expected. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Prolonged use of opioid analgesics during pregnancy may cause neonatal opioid withdrawal syndrome. Available data with Alfentanil HCl Injection in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage.
In animal reproduction studies, alfentanil reduced pup birth weights and increased pup mortality when administered to pregnant rats during gestation and throughout lactation at 9 times the human dose of 335 mcg/kg per procedure. Alfentanil was embryocidal when administered to pregnant rabbits during organogenesis at 72.6 times the human dose of 335 mcg/kg per procedure. No malformations were noted in rats or rabbits treated with alfentanil during organogenesis [see Data]. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Prolonged use of opioid analgesics during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth.
Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn. Observe newborns for symptoms of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions (5.3)].
Labor or Delivery
Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate. Alfentanil HCl Injection is not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including Alfentanil HCl Injection, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Data
Animal Data
Pregnant rats were treated with intravenous alfentanil doses of 0.08, 0.31, or 1.25 mg/kg/day (2.3, 9, or 36.6 times the human total dose of 335 mcg/kg based on body surface area, respectively). No malformations or embryotoxic effects were noted despite maternal toxicity (increased mortality in the mid- and high-dose group).
Pregnant rabbits were treated with intravenous alfentanil doses of 0.08, 0.31, or 1.25 mg/kg/day (4.6, 18, or 72.6 times the human total dose of 335 mcg/kg based on body surface area, respectively). Decreased live fetuses per litter and decreased litter size in the high dose group were noted in the presence of maternal toxicity (decreased body weight gain and mortality in the high-dose group).
No evidence of malformations or adverse effects on the fetus was reported in a published study in which pregnant rats were administered 8 mg/kg/day alfentanil (232 times the human dose of 335 mcg/kg/day based on body surface area) continuously from Gestation Day 5 through Gestation Day 20 via subcutaneously implanted osmotic minipumps.
Pregnant rats were treated intravenously with alfentanil 0.08, 0.31, or 1.25 mg/kg/day (2.3, 9, or 36.6 times the human total dose of 335 mcg/day based on body surface area, respectively) during gestation and throughout lactation. Reduced birth weights and decreased pup survival were noted in the mid- and high-dose groups in the presence of maternal toxicity (increased mortality in the mid- and high-dose groups).
8.2 Lactation
Risk Summary
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Alfentanil HCl Injection and any potential adverse effects on the breastfed infant from Alfentanil HCl Injection or from the underlying maternal condition.
Clinical Considerations
Infants exposed to Alfentanil HCl Injection through breast milk should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast- feeding is stopped.
8.3 Females and Males of Reproductive Potential
Infertility
Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6.2)].
8.4 Pediatric Use
Adequate data to support the use of Alfentanil HCl Injection in children under the age of 12 years of age are not presently available.
8.5 Geriatric Use
In one clinical trial, the dose of alfentanil required to produce anesthesia, as determined by appearance of delta waves in EEG, was 40% lower in geriatric patients than that needed in healthy young patients.
The initial dose of Alfentanil HCl Injection should be appropriately reduced in elderly. Patients over the age of 65 have been found to have reduced plasma clearance and extended terminal elimination which may prolong postoperative recovery.
Elderly patients (aged 65 years or older) may have increased sensitivity to alfentanil. In general, use caution when selecting a dosage for an elderly patient, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of Alfentanil HCl Injection slowly in geriatric patients [see Warnings and Precautions (5.6)].
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Hepatic Impairment
Alfentanil HCl Injection should be administered with caution patients with liver dysfunction because of the extensive hepatic metabolism. Reduce the dosage as needed and monitor closely for signs of respiratory depression, sedation, and hypotension.
8.7 Renal Impairment
Alfentanil HCl Injection should be administered with caution to patients with kidney dysfunction because of the renal excretion of alfentanil HCl and its metabolites. Reduce the dosage as needed and monitor for signs of respiratory depression, sedation, and hypotension.
8.8 Respiratory Impairment
Alfentanil HCl Injection should be used with caution in patients with pulmonary disease, decreased respiratory reserve, or potentially compromised respiration, in such patients opioids may additionally decrease respiratory drive and increase airway resistance. During anesthesia, this can be managed by assisted or controlled respiration.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Alfentanil HCl Injection contains alfentanil, a Schedule II controlled substance.
9.2 Abuse
Alfentanil HCl Injection contains alfentanil, a substance with a high potential for abuse similar to other opioids including morphine, sufentanil etc. Alfentanil HCl Injection can be abused and is subject to misuse, addiction, and criminal diversion [see Warnings and Precautions (5.1)].
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
Alfentanil HCl Injection, like other opioids, can be diverted for non-medical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests, as required by state and federal law, is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Risks Specific to Abuse of Sufentanil Citrate Injection
Abuse of Sufentanil Citrate Injection poses a risk of overdose and death. The risk is increased with concurrent use of Sufentanil Citrate Injection with alcohol and other central nervous system depressants.
Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
9.3 Dependence
Both tolerance and physical dependence can develop during chronic opioid therapy. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence results in withdrawal symptoms after abrupt discontinuation or a significant dosage reduction of a drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone, nalmefene), mixed agonist/ antagonist analgesics (pentazocine, butorphanol, nalbuphine), or partial agonists (buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage.
Alfentanil HCl Injection should not be abruptly discontinued [see Dosage and Administration (2.13)]. If Alfentanil HCl Injection is abruptly discontinued in a physically-dependent patient, a withdrawal syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal signs [see Use in Specific Populations (8.1)].
-
10 OVERDOSAGE
Clinical Presentation
Acute overdose with Alfentanil HCl Injection can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology (12.2)].
Treatment of Overdose
In case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques. If respiratory depression is associated with muscular rigidity, a neuromuscular blocking agent may be required to facilitate assisted or controlled ventilation. Intravenous fluids and vasoactive agents may be required to manage hemodynamic instability.
The opioid antagonists, naloxone or nalmefene, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to alfentanil overdose, administer an opioid antagonist. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to alfentanil overdose.
Because the duration of opioid reversal is expected to be less than the duration of action of alfentanil in Alfentanil HCl Injection, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product's prescribing information.
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
-
11 DESCRIPTION
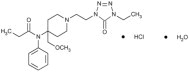
Alfentanil HCl Injection, contains alfentanil, an opioid agonist, chemically designated as N- [1- [2-(4-ethyl-4,5-dihydro-5-oxo-1H-
tetrazol-1- yl) ethyl]-4-(methoxymethyl)-4-piperidinyl]-N-phenylpropanamide monohydrochloride (1:1) with a molecular weight of 452.98 and an n-octanol: water partition coefficient of 128:1 at pH 7.4. The structural formula of Alfentanil HCl is:
Alfentanil HCl Injection, USP is a sterile, non-pyrogenic, preservative free aqueous solution containing alfentanil hydrochloride equivalent to 500 mcg per mL of alfentanil base for intravenous injection. The solution, which contains sodium chloride for isotonicity, has a pH range of 4.0 to 6.0. Each mL contains:
Active: Alfentanil base 500 mcg.
Inactive: Sodium Chloride 9 mg and Water for Injection q.s.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Alfentanil HCl Injection is an opioid agonist. The principal actions of therapeutic value are analgesia and sedation.
12.2 Pharmacodynamics
Effects on the Central Nervous System
Alfentanil produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
Alfentanil causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Alfentanil causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Alfentanil produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes and sweating and/or orthostatic hypotension.
Effects on the Endocrine System
Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon [see Adverse Reactions (6.2)].
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions (6.2)].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
In one study involving 15 patients administered alfentanil with nitrous oxide/oxygen, a narrow range of plasma alfentanil concentrations, approximately 310 to 340 ng/mL, was shown to provide adequate anesthesia for intra-abdominal surgery, while lower concentrations, approximately 190 ng/mL, blocked responses to skin closure. Plasma concentrations between 100 to 200 ng/mL provided adequate anesthesia for superficial surgery.
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids [see Dosage and Administration (2.1)]. The minimum effective analgesic concentration of alfentanil for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome and/or the development of analgesic tolerance.
Concentration–Adverse Reaction Relationships
There is a relationship between increasing alfentanil plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.1, 2.2)].
12.3 Pharmacokinetics
Alfentanil HCl Injection is administered intravenously for the induction of analgesia and anesthesia.
Absorption
The onset of the anesthetic action is immediate when Alfentanil HCl Injection is administered intravenously.
Distribution
The pharmacokinetics of alfentanil can be described as a three-compartment model with sequential distribution half-lives of 1 and 14 minutes. Alfentanil has an apparent volume of distribution of 0.4 to 1 L/kg, with an average plasma clearance of 5 mL/kg/min. Plasma protein binding of alfentanil is approximately 92%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of alfentanil have not been conducted.
Mutagenesis
Alfentanil was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test) or clastogenic in the in vivo micronucleus assay.
Impairment of Fertility
Female rats were treated with intravenous alfentanil beginning 14 days prior to mating and throughout gestation with doses of 0.08, 0.31, or 1.25 mg/kg (2.3, 9, or 36.3 times the human daily dose of 335 mcg/day based on body surface area, respectively) prior to mating with non-dosed males. Maternal toxicity was noted in all animals (decreased weight gain in all groups and mortality in the two highest dose groups). There was no clear effect on female fertility.
Male rats were treated intravenously with doses of 0.08, 0.31, or 1.25 mg/kg (2.3, 9, or 36.3 times the human daily dose of 335 mcg/day based on body surface area, respectively) beginning 56 days prior to mating with non-dosed females. There was reduced pregnancy rate in the untreated females mated to the high dose males; however, there was also paternal toxicity was noted in all animals (decreased weight gain in all groups and mortality in the two highest dose groups).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Alfentanil HCl Injection, USP for intravenous use is a sterile, aqueous, preservative free solution. Each mL contains: Active: Alfentanil base 500 mcg. Inactives: Sodium Chloride 9 mg and WFI q.s.
Alfentanil HCl Injection, USP available as:
NDC: 17478-067-02, 2 mL Ampule in packages of 10
NDC: 17478-067-05, 5 mL Ampule in packages of 10
NDC: 17478-067-10, 10 mL Ampule in packages of 5
NDC: 17478-067-20, 20 mL Ampule in packages of 5
Alfentanil HCl Injection, USP is supplied in individually sealed dosage forms which pose no known risk to health care providers having incidental contact. Accidental dermal exposure to alfentanil should be treated by rinsing the affected area with water.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-067-02 2 mL Ampule
Alfentanil HCl CII
Injection, USP
1000 mcg/2 mL (500 mcg/mL)
Alfentanil base
Rx only
May be habit forming.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-067-02 10 Ampules (2 mL each)
Alfentanil HCl CII
Injection, USP
1000 mcg/2 mL (500 mcg/mL)
FOR INTRAVENOUS USE
Rx only Akorn Logo
-
INGREDIENTS AND APPEARANCE
ALFENTANIL HYDROCHLORIDE
alfentanil hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-067 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alfentanil Hydrochloride (UNII: 11S92G0TIW) (Alfentanil - UNII:1N74HM2BS7) Alfentanil 500 ug in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-067-02 10 in 1 CARTON 02/01/2010 1 2 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 17478-067-05 10 in 1 CARTON 02/01/2010 2 5 mL in 1 AMPULE; Type 0: Not a Combination Product 3 NDC: 17478-067-10 5 in 1 CARTON 02/01/2010 3 10 mL in 1 AMPULE; Type 0: Not a Combination Product 4 NDC: 17478-067-20 5 in 1 CARTON 02/01/2010 4 20 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019353 02/01/2010 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 063434679 PACK(17478-067) , LABEL(17478-067) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE(17478-067) , ANALYSIS(17478-067) , STERILIZE(17478-067)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.