LINZESS- linaclotide capsule, gelatin coated
Linzess by
Drug Labeling and Warnings
Linzess by is a Prescription medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LINZESS safely and effectively. See full prescribing information for LINZESS.
LINZESS® (linaclotide) capsules, for oral use
Initial U.S. Approval: 2012
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
See full prescribing information for complete boxed warning.
-
LINZESS is contraindicated in patients less than 6 years of age; in neonatal mice, linaclotide caused deaths due to dehydration. (4, 8.4)
-
Avoid use of LINZESS in patients 6 years to less than 18 years of age. (5.1, 8.4)
- The safety and effectiveness of LINZESS have not been established in patients less than 18 years of age (8.4).
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
The recommended dosage in adults is:
- IBS-C: 290 mcg orally once daily. (2.1)
- CIC: 145 mcg orally once daily or 72 mcg orally once daily based on individual presentation or tolerability. (2.1)
Administration Instructions (2.2):
- Take on empty stomach at least 30 minutes prior to first meal of the day.
- Do not crush or chew LINZESS capsule or capsule contents.
- For patients who have difficulty swallowing capsules whole or those with a nasogastric or gastrostomy tube, see full prescribing information for instructions for opening the capsule and administering with applesauce or water.
DOSAGE FORMS AND STRENGTHS
Capsules: 72 mcg, 145 mcg and 290 mcg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Diarrhea: Patients may experience severe diarrhea. If severe diarrhea occurs,suspend dosing and rehydrate the patient. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (≥2%) reported in IBS-C or CIC patients are: diarrhea, abdominal pain, flatulence and abdominal distension. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2018
-
LINZESS is contraindicated in patients less than 6 years of age; in neonatal mice, linaclotide caused deaths due to dehydration. (4, 8.4)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Dehydration in Pediatric Patients
5.2 Diarrhea
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Irritable Bowel Syndrome with Constipation (IBS-C)
14.2 Chronic Idiopathic Constipation (CIC)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
-
LINZESS is contraindicated in patients less than 6 years of age; in nonclinical studies in neonatal mice, administration of a single, clinically relevant adult oral dose of linaclotide caused deaths due to dehydration [see Contraindications (4), Use in Specific Populations (8.4)].
-
Avoid use of LINZESS in patients 6 years to less than 18 years of age [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
- The safety and effectiveness of LINZESS have not been established in patients less than 18 years of age [see Use in Specific Populations (8.4)].
-
LINZESS is contraindicated in patients less than 6 years of age; in nonclinical studies in neonatal mice, administration of a single, clinically relevant adult oral dose of linaclotide caused deaths due to dehydration [see Contraindications (4), Use in Specific Populations (8.4)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Irritable Bowel Syndrome with Constipation (IBS-C)
The recommended dosage of LINZESS is 290 mcg orally once daily.
Chronic Idiopathic Constipation (CIC)
The recommended dosage of LINZESS is 145 mcg orally once daily. A dosage of 72 mcg once daily may be used based on individual presentation or tolerability.
2.2 Preparation and Administration Instructions
- Take LINZESS on an empty stomach, at least 30 minutes prior to the first meal of the day
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take 2 doses at the same time.
- Do not crush or chew LINZESS capsule or capsule contents.
- Swallow LINZESS capsule whole.
- For adult patients with swallowing difficulties, LINZESS capsules can be opened and administered orally in either applesauce or with water or administered with water via a nasogastric or gastrostomy tube. Sprinkling of LINZESS beads on other soft foods or in other liquids has not been tested.
Oral Administration in Applesauce:
- Place one teaspoonful of room-temperature applesauce into a clean container.
- Open the capsule.
- Sprinkle the entire contents (beads) on applesauce.
- Consume the entire contents immediately. Do not chew the beads. Do not store the bead-applesauce mixture for later use.
Oral Administration in Water:
- Pour approximately 30 mL of room-temperature bottled water into a clean cup.
- Open the capsule
- Sprinkle the entire contents (beads) into the water
- Gently swirl beads and water for at least 20 seconds.
- Swallow the entire mixture of beads and water immediately.
- Add another 30 mL of water to any beads remaining in cup, swirl for 20 seconds, and swallow immediately.
- Do not store the bead-water mixture for later use.
Note: The drug is coated on the surface of the beads and will dissolve off the beads into the water. The beads will remain visible and will not dissolve. Therefore, it is not necessary to consume all the beads to deliver the complete dose.
Administration with Water via a Nasogastric or Gastrostomy Tube:
- Open the capsule and empty the beads into a clean container with 30 mL of room-temperature bottled water.
- Mix by gently swirling beads for at least 20 seconds
- Draw-up the beads and water mixture into an appropriately sized catheter-tipped syringe and apply rapid and steady pressure (10 mL/10 seconds) to dispense the syringe contents into the tube.
- Add another 30 mL of water to any beads remaining in the container and repeat the process
- After administering the bead-water mixture, flush nasogastric/ gastrostomy tube with a minimum of 10 mL of water.
Note: It is not necessary to flush all the beads through to deliver the complete dose.
- Take LINZESS on an empty stomach, at least 30 minutes prior to the first meal of the day
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Dehydration in Pediatric Patients
LINZESS is contraindicated in patients less than 6 years of age. The safety and effectiveness of LINZESS in patients less than 18 years of age have not been established. In neonatal mice (human age equivalent of approximately 0 to 28 days), linaclotide increased fluid secretion as a consequence of GC-C agonism resulting in mortality within the first 24 hours due to dehydration. Due to increased intestinal expression of GC-C, patients less than 6 years of age may be more likely than patients 6 years of age and older to develop severe diarrhea and its potentially serious consequences.
Avoid use of LINZESS in pediatric patients 6 years to less than 18 years of age. Although there were no deaths in older juvenile mice, given the deaths in young juvenile mice and the lack of clinical safety and efficacy data in pediatric patients, avoid the use of LINZESS in pediatric patients 6 years to less than 18 years of age [see Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.4)].
5.2 Diarrhea
Diarrhea was the most common adverse reaction of LINZESS-treated patients in the pooled IBS-C and CIC double-blind placebo-controlled trials. The incidence of diarrhea was similar between the IBS-C and CIC populations. Severe diarrhea was reported in 2% of 145 mcg and 290 mcg LINZESS-treated patients, and in <1% of 72 mcg LINZESS-treated CIC patients [see Adverse Reactions (6.1)].
In post-marketing experience, severe diarrhea associated with dizziness, syncope, hypotension and electrolyte abnormalities (hypokalemia and hyponatremia) requiring hospitalization or intravenous fluid administration have been reported in patients treated with LINZESS.
If severe diarrhea occurs, suspend dosing and rehydrate the patient.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Exposure in clinical development included approximately 2570, 2040, and 1220 patients with either IBS-C or CIC treated with LINZESS for 6 months or longer, 1 year or longer, and 18 months or longer, respectively (not mutually exclusive).
Demographic characteristics were comparable between treatment groups in all studies [see Clinical Studies (14)].
Irritable Bowel Syndrome with Constipation (IBS-C)
Most Common Adverse Reactions
The data described below reflect exposure to LINZESS in the two placebo-controlled clinical trials involving 1605 adult patients with IBS-C (Trials 1 and 2). Patients were randomized to receive placebo or 290 mcg LINZESS once daily on an empty stomach for up to 26 weeks. Table 1 provides the incidence of adverse reactions reported in at least 2% of IBS-C patients in the LINZESS treatment group and at an incidence that was greater than in the placebo group.
Table 1: Most Common Adverse Reactionsa in Two Placebo-Controlled Trials (1 and 2) in Patients with IBS-C Adverse Reactions
LINZESS
290 mcg
[N=807]
%Placebo
[N=798]
%Gastrointestinal
Diarrhea
Abdominal painb
Flatulence
Abdominal distension20
7
4
23
5
2
1Infections and Infestations
Viral Gastroenteritis3 1 Nervous System Disorders
Headache4 3 a: Reported in at least 2% of LINZESS-treated patients and at an incidence greater than placebo
b: “Abdominal pain” term includes abdominal pain, upper abdominal pain, and lower abdominal pain.
Diarrhea
Diarrhea was the most commonly reported adverse reaction of the LINZESS-treated patients in the pooled IBS-C pivotal placebo-controlled trials. In these trials, 20% of LINZESS-treated patients reported diarrhea compared to 3% of placebo-treated patients. Severe diarrhea was reported in 2% of the LINZESS-treated patients versus less than 1% of the placebo-treated patients, and 5% of LINZESS-treated patients discontinued due to diarrhea vs less than 1% of placebo-treated patients. The majority of reported cases of diarrhea started within the first 2 weeks of LINZESS treatment [see Warnings and Precautions (5.2)].
Adverse Reactions Leading to Discontinuation
In placebo-controlled trials in patients with IBS-C, 9% of patients treated with LINZESS and 3% of patients treated with placebo discontinued prematurely due to adverse reactions. In the LINZESS treatment group, the most common reasons for discontinuation due to adverse reactions were diarrhea (5%) and abdominal pain (1%). In comparison, less than 1% of patients in the placebo group withdrew due to diarrhea or abdominal pain.
Adverse Reactions Leading to Dose Reductions
In the open-label, long-term trials, 2147 patients with IBS-C received 290 mcg of LINZESS daily for up to 18 months. In these trials, 29% of patients had their dose reduced or suspended secondary to adverse reactions, the majority of which were diarrhea or other GI adverse reactions.
Less Common Adverse Reactions
Defecation urgency, fecal incontinence, vomiting, and gastroesophagal reflux disease were reported in <2% of patients in the LINZESS treatment group and at an incidence greater than in the placebo treatment group.
Chronic Idiopathic Constipation (CIC)
Most Common Adverse Reactions
The data described below reflect exposure to LINZESS in the two double-blind placebo-controlled clinical trials of 1275 adult patients with CIC (Trials 3 and 4). Patients were randomized to receive placebo or 145 mcg LINZESS or 290 mcg LINZESS once daily on an empty stomach, for at least 12 weeks. Table 2 provides the incidence of adverse reactions reported in at least 2% of CIC patients in the 145 mcg LINZESS treatment group and at an incidence that was greater than in the placebo treatment group.
Table 2: Most Common Adverse Reactionsa in the Two Placebo-controlled Trials (3 and 4) in Patients with CIC Adverse Reactions LINZESS
145 mcg
[N=430]
%Placebo
[N=423]
%Gastrointestinal
Diarrhea
Abdominal painb
Flatulence
Abdominal distension16
7
6
35
6
5
2Infections and Infestations
Upper respiratory tract infection
Sinusitis5
34
2a: Reported in at least 2% of LINZESS-treated patients and at an incidence greater than placebo
b: “Abdominal pain” term includes abdominal pain, upper abdominal pain, and lower abdominal pain.
The safety of a 72 mcg dose was evaluated in an additional placebo-controlled trial in which 1223 patients were randomized to LINZESS 72 mcg, 145 mcg, or placebo once daily for 12 weeks (Trial 5).
In Trial 5, adverse reactions that occurred at a frequency of ≥ 2% in LINZESS-treated patients (n=411 in each LINZESS 72 mcg and 145 mcg group) and at a higher rate than placebo (n=401) were:
- Diarrhea (LINZESS 72 mcg 19%; LINZESS 145 mcg 22%; placebo 7%)
- Abdominal distension (LINZESS 72 mcg 2%; LINZESS 145 mcg 1%; placebo < 1%)
Diarrhea
This section summarizes information from Trials 3 and 4 (pooled) and Trial 5 regarding diarrhea, the most commonly reported adverse reaction reported in LINZESS-treated patients in CIC placebo-controlled studies.
In all trials, the majority of reported cases of diarrhea started within the first 2 weeks of LINZESS treatment.
Severe diarrhea was reported in less than 1% of the 72 mcg LINZESS-treated patients (Trial 5), in 2% of the 145 mcg LINZESS-treated patients (Trials 3 and 4; Trial 5), and less than 1% of the placebo-treated patients (Trials 3, 4, and 5) [see Warnings and Precautions (5.2)].
Adverse Reactions Leading to Discontinuation
In placebo-controlled trials in patients with CIC, 3% of patients treated with 72 mcg (Trial 5) and between 5% (Trial 5) and 8% (Trials 3 and 4) of patients treated with 145 mcg of LINZESS discontinued prematurely due to adverse reactions compared to between less than 1% (Trial 5) and 4% (Trials 3 and 4) of patients treated with placebo.
In patients treated with 72 mcg LINZESS the most common reason for discontinuation due to adverse reactions was diarrhea (2% in Trial 5) and in patients treated with 145 mcg LINZESS, the most common reasons for discontinuation due to adverse reactions were diarrhea (3% in Trial 5 and 5% in Trials 3 and 4) and abdominal pain (1% in Trials 3 and 4). In comparison, less than 1% of patients in the placebo group withdrew due to diarrhea or abdominal pain (Trials 3 and 4; Trial 5).
Adverse Reactions Leading to Dose Reductions
In the open-label, long-term trials, 1129 patients with CIC received 290 mcg of LINZESS daily for up to 18 months. In these trials, 27% of patients had their dose reduced or suspended secondary to adverse reactions, the majority of which were diarrhea or other GI adverse reactions.
Less Common Adverse Reactions
Defecation urgency, fecal incontinence, dyspepsia, and viral gastroenteritis, were reported in less than 2% of patients in the LINZESS treatment group and at an incidence greater than placebo treatment group.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of LINZESS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hematochezia, rectal hemorrhage, nausea, and allergic reactions, urticaria or hives.
- Diarrhea (LINZESS 72 mcg 19%; LINZESS 145 mcg 22%; placebo 7%)
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Linaclotide and its active metabolite are negligibly absorbed systemically following oral administration [see Clinical Pharmacology (12.3)], and maternal use is not expected to result in fetal exposure to the drug. The available data on LINZESS use in pregnant women are not sufficient to inform any drug-associated risk for major birth defects and miscarriage. In animal developmental studies, no effects on embryo-fetal development were observed with oral administration of linaclotide in rats and rabbits during organogenesis at doses much higher than the maximum recommended human dosage. Severe maternal toxicity associated with effects on fetal morphology were observed in mice [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
The potential for linaclotide to cause harm to embryo-fetal development was studied in rats, rabbits and mice. In pregnant mice, oral dose levels of at least 40,000 mcg/kg/day given during organogenesis produced severe maternal toxicity including death, reduction of gravid uterine and fetal weights, and effects on fetal morphology. Oral doses of 5,000 mcg/kg/day did not produce maternal toxicity or any adverse effects on embryo-fetal development in mice. Oral administration of up to 100,000 mcg/kg/day in rats and 40,000 mcg/kg/day in rabbits during organogenesis produced no maternal toxicity and no effects on embryo-fetal development. Additionally, oral administration of up to 100,000 mcg/kg/day in rats during organogenesis through lactation produced no developmental abnormalities or effects on growth, learning and memory, or fertility in the offspring through maturation.
The maximum recommended human dose is approximately 5 mcg/kg/day, based on a 60-kg body weight. Limited systemic exposure to linaclotide was achieved in animals during organogenesis (AUC = 40, 640, and 25 nghr/mL in rats, rabbits, and mice, respectively, at the highest dose levels). Linaclotide and its active metabolite are not measurable in human plasma following administration of the recommended clinical dosages. Therefore, animal and human doses should not be compared directly for evaluating relative exposure.
8.2 Lactation
Risk Summary
There is no information regarding the presence of linaclotide in human milk, or on its effects on milk production or the breastfed infant. No lactation studies in animals have been conducted. Linaclotide and its active metabolite are negligibly absorbed systemically following oral administration [see Clinical Pharmacology (12.3)]. It is unknown whether the negligible systemic absorption of linaclotide by adults will result in a clinically relevant exposure to breastfed infants. Exposure to linaclotide in breastfed infants has the potential for serious adverse effects [see Use in Specific Populations (8.4)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LINZESS and any potential adverse effects on the breastfed infant from LINZESS or from the underlying maternal condition.
8.4 Pediatric Use
LINZESS is contraindicated in patients less than 6 years of age. Avoid use of LINZESS in patients 6 years to less than 18 years of age [see Contraindications (4), Warnings and Precautions (5.1)]. The safety and effectiveness of LINZESS in patients less than 18 years of age have not been established.
In nonclinical studies, deaths occurred within 24 hours in neonatal mice (human age equivalent of approximately 0 to 28 days) following oral administration of linaclotide, as described below in Juvenile Animal Toxicity Data. Because of increased intestinal expression of GC-C, patients less than 6 years of age may be more likely than patients 6 years of age and older to develop diarrhea and its potentially serious consequences. LINZESS is contraindicated in patients less than 6 years of age.
Given the deaths in young juvenile mice and the lack of clinical safety and efficacy data in pediatric patients, avoid the use of LINZESS in patients 6 years to less than 18 years of age.
Juvenile Animal Toxicity Data
In toxicology studies in neonatal mice, oral administration of linaclotide at 10 mcg/kg/day caused deaths on post-natal day 7 (human age equivalent of approximately 0 to 28 days). These deaths were due to rapid and severe dehydration produced by significant fluid shifts into the intestinal lumen resulting from GC-C agonism in neonatal mice [see Contraindications (4) and Warnings and Precautions (5.1)].
Tolerability to linaclotide increases with age in juvenile mice. In 2-week-old mice, linaclotide was well tolerated at a dose of 50 mcg/kg/day, but deaths occurred after a single oral dose of 100 mcg/kg. In 3-week-old mice, linaclotide was well tolerated at 100 mcg/kg/day, but deaths occurred after a single oral dose of 600 mcg/kg.
8.5 Geriatric Use
Irritable Bowel Syndrome with Constipation (IBS-C)
Of 1605 IBS-C patients in the placebo-controlled clinical studies of LINZESS, 85 (5%) were 65 years of age and over, while 20 (1%) were 75 years and over. Clinical studies of LINZESS did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.Chronic Idiopathic Constipation (CIC)
Of 2498 CIC patients in the placebo-controlled clinical studies of LINZESS (Trials 3, 4, and 5), 273 (11%) were 65 years of age and over, while 56 (2%) were 75 years and over. Clinical studies of LINZESS did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. - 10 OVERDOSAGE
-
11 DESCRIPTION
LINZESS (linaclotide) is a guanylate cyclase-C (G-CC) agonist. Linaclotide is a 14-amino acid peptide with the following chemical name: L-cysteinyl-L-cysteinyl-L-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-L-asparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threonyl-glycyl-L-cysteinyl-L-tyrosine, cyclic (1-6), (2-10), (5-13)-tris (disulfide).
The molecular formula of linaclotide is C59H79N15O21S6 and its molecular weight is 1526.8. The amino acid sequence for linaclotide is shown below:
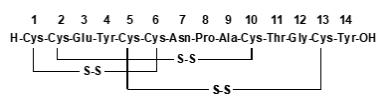
Linaclotide is an amorphous, white to off-white powder. It is slightly soluble in water and aqueous sodium chloride (0.9%). LINZESS contains linaclotide-coated beads in hard gelatin capsules. LINZESS is available as 72 mcg, 145 mcg and 290 mcg capsules for oral administration.
The inactive ingredients of LINZESS 72 mcg capsules include: calcium chloride dihydrate, L-histidine, microcrystalline cellulose, polyvinyl alcohol, and talc. The components of the capsule shell include gelatin and titanium dioxide.
The inactive ingredients of LINZESS 145 mcg and 290 mcg capsules include: calcium chloride dihydrate, hypromellose, L-leucine, and microcrystalline cellulose. The components of the capsule shell include gelatin and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Linaclotide is structurally related to human guanylin and uroguanylin and functions as a guanylate cyclase-C (GC-C) agonist. Both linaclotide and its active metabolite bind to GC-C and act locally on the luminal surface of the intestinal epithelium. Activation of GC-C results in an increase in both intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP). Elevation in intracellular cGMP stimulates secretion of chloride and bicarbonate into the intestinal lumen, mainly through activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, resulting in increased intestinal fluid and accelerated transit. In animal models, linaclotide has been shown to both accelerate GI transit and reduce intestinal pain.
In an animal model of visceral pain, linaclotide reduced abdominal muscle contraction and decreased the activity of pain-sensing nerves by increasing extracellular cGMP.
12.2 Pharmacodynamics
Food Effect
Taking LINZESS immediately after the high fat breakfast resulted in looser stools and a higher stool frequency compared with taking it in the fasted state [see Dosage and Administration (2.1, 2.2)]. In clinical trials, LINZESS was administered on an empty stomach, at least 30 minutes before breakfast.
12.3 Pharmacokinetics
Absorption
LINZESS is minimally absorbed with negligible systemic availability following oral administration. Concentrations of linaclotide and its active metabolite in plasma are below the limit of quantitation after oral doses of 145 mcg or 290 mcg were administered. Therefore, standard pharmacokinetic parameters such as area under the curve (AUC), maximum concentration (Cmax), and half-life (t½) cannot be calculated.
Food Effect
Neither linaclotide nor its active metabolite were detected in the plasma following administration of LINZESS 290 mcg once daily for 7 days both in the non-fed and fed state in healthy subjects.
Distribution
Given that linaclotide plasma concentrations following recommended oral doses are not measurable, linaclotide is not expected to be distributed to tissues to any clinically relevant extent.
Elimination
Metabolism
Linaclotide is metabolized within the gastrointestinal tract to its principal, active metabolite by loss of the terminal tyrosine moiety. Both linaclotide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids.
Excretion
Active peptide recovery in the stool samples of fed and fasted healthy subjects following administration of LINZESS 290 mcg once daily for seven days averaged about 5% (fasted) and about 3% (fed) and all of it as the active metabolite.
Specific Populations
Renal and Hepatic Impairment
Renal or hepatic impairment is not expected to affect the clearance of linaclotide or the active metabolite because linaclotide metabolism occurs within the gastrointestinal tract and plasma concentrations are not measurable in plasma following administration of the recommended dosage.
Drug Interaction Studies
No drug-drug interaction studies have been conducted with LINZESS. Systemic exposures of drug and active metabolite are negligible following oral administration.
Linaclotide does not interact with the cytochrome P450 enzyme system based on the results of in vitro studies. In addition, linaclotide does not interact with common efflux and uptake transporters (including the efflux transporter P-glycoprotein (P-gp)). Based on these in vitro data no drug drug interactions through modulation of CYP enzymes or common transporters are anticipated.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In 2-year carcinogenicity studies, linaclotide was not tumorigenic in rats at doses up to 3500 mcg/kg/day or in mice at doses up to 6000 mcg/kg/day. The maximum recommended human dose is approximately 5 mcg/kg/day based on a 60-kg bodyweight. Limited systemic exposure to linaclotide and its active metabolite was achieved at the tested dose levels in animals, whereas no detectable exposure occurred in humans. Therefore, animal and human doses should not be compared directly for evaluating relative exposure.
Mutagenesis
Linaclotide was not genotoxic in an in vitro bacterial reverse mutation (Ames) assay or in the in vitro chromosomal aberration assay in cultured human peripheral blood lymphocytes.
Impairment of Fertility
Linaclotide had no effect on fertility or reproductive function in male and female rats at oral doses of up to 100,000 mcg/kg/day.
-
14 CLINICAL STUDIES
14.1 Irritable Bowel Syndrome with Constipation (IBS-C)
The efficacy of LINZESS for the management of symptoms of IBS-C was established in two double-blind, placebo-controlled, randomized, multicenter trials in adult patients (Trials 1 and 2). A total of 800 patients in Trial 1 and 804 patients in Trial 2 [overall mean age of 44 years (range 18 to 87 years), 90% female, 77% white, 19% black, and 12% Hispanic] received treatment with LINZESS 290 mcg or placebo once daily and were evaluated for efficacy. All patients met Rome II criteria for IBS and were required, during the 2-week baseline period, to meet the following criteria:
- a mean abdominal pain score of at least 3 on a 0-to-10-point numeric rating scale
- less than 3 complete spontaneous bowel movements (CSBMs) per week [a CSBM is a spontaneous bowel movement (SBM) that is associated with a sense of complete evacuation; a SBM is a bowel movement occurring in the absence of laxative use], and
- less than or equal to 5 SBMs per week.
The trial designs were identical through the first 12 weeks, and thereafter differed only in that Trial 1 included a 4-week randomized withdrawal (RW) period, and Trial 2 continued for 14 additional weeks (total of 26 weeks) of double-blind treatment. During the trials, patients were allowed to continue stable doses of bulk laxatives or stool softeners but were not allowed to take laxatives, bismuth, prokinetic agents, or other drugs to treat IBS-C or chronic constipation.
Efficacy of LINZESS was assessed using overall responder analyses and change-from-baseline endpoints. Results for endpoints were based on information provided daily by patients in diaries.
The 4 primary efficacy responder endpoints were based on a patient being a weekly responder for either at least 9 out of the first 12 weeks of treatment or at least 6 out of the first 12 weeks of treatment. For the 9 out of 12 weeks combined primary responder endpoint, a patient had to have at least a 30% reduction from baseline in mean abdominal pain, at least 3 CSBMs and an increase of at least 1 CSBM from baseline, all in the same week, for at least 9 out of the first 12 weeks of treatment. Each of the 2 components of the 9 out of 12 weeks combined responder endpoint, abdominal pain and CSBMs, was also a primary endpoint.
For the 6 out of 12 weeks combined primary responder endpoint, a patient had to have at least a 30% reduction from baseline in mean abdominal pain and an increase of at least 1 CSBM from baseline, all in the same week, for at least 6 out of the first 12 weeks of treatment. To be considered a responder for this analysis, patients did not have to have at least 3 CSBMs per week.
The efficacy results for the 9 out of 12 weeks and the 6 out of 12 weeks responder endpoints are shown in Tables 3 and 4, respectively. In both trials, the proportion of patients who were responders to LINZESS 290 mcg was statistically significantly higher than with placebo.
Table 3: Efficacy Responder Rates in the Two Placebo-controlled IBS-C Trials: at Least 9 Out of 12 Weeks Trial 1 Trial 2 LINZESS
290 mcg
(N=405)Placebo
(N=395)Treatment Difference
[95% CI]LINZESS
290 mcg
(N=401)Placebo
(N=403)Treatment Difference
[95% CI]Combined Responder*
(Abdominal Pain and CSBM Responder)12% 5% 7%
[3.2%, 10.9%]13% 3% 10%
[6.1%, 13.4%]Abdominal Pain Responder*
(≥ 30% Abdominal Pain Reduction)34% 27% 7%
[0.9%, 13.6%]39% 20% 19%
[13.2%, 25.4%]CSBM Responder*
(≥ 3 CSBMs and Increase ≥1 CSBM from Baseline)20% 6% 13%
[8.6%, 17.7%]18% 5% 13%
[8.7%, 17.3%]* Primary Endpoints
Note: Analyses based on first 12 weeks of treatment for both Trials 1 and 2
CI =Confidence IntervalTable 4: Efficacy Responder Rates in the Two Placebo-controlled IBS-C Trials: at Least 6 Out of 12 Weeks Trial 1 Trial 2 LINZESS
290 mcg
(N=405)Placebo
(N=395)Treatment Difference
[95% CI]LINZESS
290 mcg
(N=401)Placebo
(N=403)Treatment Difference
[95% CI]Combined Responder*
(Abdominal Pain and CSBM Responder)34% 21% 13%
[6.5%, 18.7%]34% 14% 20%
[14.0%, 25.5%]Abdominal Pain Responder**
(≥ 30% Abdominal Pain Reduction)50% 37% 13%
[5.8%, 19.5%]49% 34% 14%
[7.6%, 21.1%]CSBM Responder**
(Increase ≥ 1 CSBM from Baseline)49% 30% 19%
[12.4%, 25.7%]48% 23% 25%
[18.7%, 31.4%]* Primary Endpoint, ** Secondary Endpoints
Note: Analyses based on first 12 weeks of treatment for both Trials 1 and 2
CI =Confidence IntervalIn each trial, improvement from baseline in abdominal pain and CSBM frequency was seen over the first 12-weeks of the treatment periods. For change from baseline in the 11-point abdominal pain scale, LINZESS 290 mcg began to separate from placebo in the first week. Maximum effects were seen at weeks 6 - 9 and were maintained until the end of the study. The mean treatment difference from placebo at week 12 was a decrease in pain score of approximately 1.0 point in both trials (using an 11-point scale). Maximum effect on CSBM frequency occurred within the first week, and for change from baseline in CSBM frequency at week 12, the difference between placebo and LINZESS was approximately 1.5 CSBMs per week in both trials.
In each trial, in addition to improvements in abdominal pain and CSBM frequency over the first 12 weeks of the treatment period, improvements were observed in the following when LINZESS was compared to placebo: SBM frequency [SBMs/week], stool consistency [as measured by the Bristol Stool Form Scale (BSFS)], and amount of straining with bowel movements [amount of time pushing or physical effort to pass stool].
During the 4-week randomized withdrawal period in Trial 1, patients who received LINZESS during the 12-week treatment period were re-randomized to receive placebo or continue treatment on LINZESS 290 mcg. In LINZESS-treated patients re-randomized to placebo, CSBM frequency and abdominal-pain severity returned toward baseline within 1 week and did not result in worsening compared to baseline. Patients who continued on LINZESS maintained their response to therapy over the additional 4 weeks. Patients on placebo who were allocated to LINZESS had an increase in CSBM frequency and a decrease in abdominal pain levels that were similar to the levels observed in patients taking LINZESS during the treatment period.
14.2 Chronic Idiopathic Constipation (CIC)
The efficacy of LINZESS for the management of symptoms of CIC was established in two double-blind, placebo-controlled, randomized, multicenter clinical trials in adult patients (Trials 3 and 4). A total of 642 patients in Trial 3 and 630 patients in Trial 4 [overall mean age of 48 years (range 18 to 85 years), 89% female, 76% white, 22% black, 10% Hispanic] received treatment with LINZESS 145 mcg, 290 mcg, or placebo once daily and were evaluated for efficacy. All patients met modified Rome II criteria for functional constipation. Modified Rome II criteria were less than 3 Spontaneous Bowel Movements (SBMs) per week and 1 of the following symptoms for at least 12 weeks, which need not be consecutive, in the preceding 12 months:
- Straining during greater than 25% of bowel movements
- Lumpy or hard stools during greater than 25% of bowel movements
- Sensation of incomplete evacuation during greater than 25% of bowel movements
Patients were also required to have less than 3 CSBMs per week and less than or equal to 6 SBMs per week during a 2-week baseline period. Patients were excluded if they met criteria for IBS-C or had fecal impaction that required emergency room treatment.
The trial designs were identical through the first 12 weeks. Trial 3 also included an additional 4-week randomized withdrawal (RW) period. During the trials, patients were allowed to continue stable doses of bulk laxatives or stool softeners but were not allowed to take laxatives, bismuth, prokinetic agents, or other drugs to treat chronic constipation.
The efficacy of LINZESS was assessed using a responder analysis and change-from-baseline endpoints. Results for endpoints were based on information provided daily by patients in diaries.
A CSBM responder in the CIC trials was defined as a patient who had at least 3 CSBMs and an increase of at least 1 CSBM from baseline in a given week for at least 9 weeks out of the 12-week treatment period. The CSBM responder rates are shown in Table 5. During the individual double-blind placebo-controlled trials, LINZESS 290 mcg did not consistently offer additional clinically meaningful treatment benefit over placebo than that observed with the LINZESS 145 mcg dose. Therefore, the 145 mcg dose is the recommended dose. Only the data for the approved 145 mcg dose of LINZESS are presented in Table 5.
In Trials 3 and 4, the proportion of patients who were CSBM responders was statistically significantly greater with the LINZESS 145 mcg dose than with placebo.
Table 5: Efficacy Responder Rates in the Two Placebo-controlled CIC Trials: at Least 9 Out of 12 Weeks Trial 3 Trial 4 LINZESS
145 mcg
(N=217)Placebo
(N=209)Treatment Difference
[95% CI]LINZESS
145 mcg
(N=213)Placebo
(N=215)Treatment Difference
[95% CI]CSBM Responder*
(≥ 3 CSBMs and Increase ≥ 1 CSBM from Baseline)20% 3% 17%
[11.0%, 22.8%]15% 6% 10%
[4.2%, 15.7%]*Primary Endpoint
CI=Confidence IntervalCSBM frequency reached maximum level during week 1 and was also demonstrated over the remainder of the 12-week treatment period in Trial 3 and Trial 4. For the mean change from baseline in CSBM frequency at week 12, the difference between placebo and LINZESS was approximately 1.5 CSBMs.
On average, patients who received LINZESS across the 2 trials had significantly greater improvements compared with patients receiving placebo in stool frequency (CSBMs/week and SBMs/week), and stool consistency (as measured by the BSFS).
In each trial, in addition to improvements in CSBM frequency over the first 12 weeks of the treatment period, improvements were observed in each of the following when LINZESS was compared to placebo: SBM frequency [SBMs/week], stool consistency [as measured by the BSFS], and amount of straining with bowel movements [amount of time pushing or physical effort to pass stool].
During the 4-week randomized withdrawal period in Trial 3, patients who received LINZESS during the 12-week treatment period were re-randomized to receive placebo or continue treatment on the same dose of LINZESS taken during the treatment period. In LINZESS-treated patients re-randomized to placebo, CSBM and SBM frequency returned toward baseline within 1 week and did not result in worsening compared to baseline. Patients who continued on LINZESS maintained their response to therapy over the additional 4 weeks. Patients on placebo who were allocated to LINZESS had an increase in CSBM and SBM frequency similar to the levels observed in patients taking LINZESS during the treatment period.
A 72 mcg dose of LINZESS was established in a randomized, double-blind, placebo-controlled, multicenter clinical trial in adult patients (Trial 5). A total of 1223 patients [overall mean age of 46 years (range 18 to 90 years), 77% female, 71% white, 24% black, 43% Hispanic] received treatment with LINZESS 72 mcg or placebo once daily and were evaluated for efficacy. All patients met modified Rome III criteria for functional constipation. Trial 5 was identical to Trials 3 and 4 through the first 12 weeks. The efficacy of the 72 mcg dose was assessed using a responder analysis where a CSBM responder was defined as a patient who had at least 3 CSBMs and an increase of at least 1 CSBM from baseline in a given week for at least 9 weeks out of the 12-week treatment period, which was the same as the one defined in Trials 3 and 4. The response rates for the CSBM responder endpoint were 13% for LINZESS 72 mcg and 5% for placebo. The difference between LINZESS 72 mcg and placebo was 9% (95% CI: 4.8%, 12.5%).
A separate analysis was performed using an alternate CSBM responder definition. In this analysis a CSBM responder was defined as a patient who had at least 3 CSBMs and an increase of at least 1 CSBM from baseline in a given week for at least 9 weeks out of the 12-week treatment period and at least 3 of the last 4 weeks of the treatment period. The response rates for the alternate CSBM responder endpoint were 12% for LINZESS 72 mcg and 5% for placebo. The difference between LINZESS 72 mcg and placebo was 8% (95% CI: 3.9%, 11.5%).
- a mean abdominal pain score of at least 3 on a 0-to-10-point numeric rating scale
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
LINZESS Capsule Strength Description Packaging NDC number 72 mcg White to off-white opaque hard gelatin capsules with gray imprint “FL 72” Bottle of 30 0456-1203-30 145 mcg White to off-white opaque hard gelatin capsules with gray imprint "FL 145" Bottle of 30 0456-1201-30 290 mcg White to off-white opaque hard gelatin capsules with gray imprint "FL 290" Bottle of 30 0456-1202-30 Storage
Store at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Keep LINZESS in the original container. Do not subdivide or repackage. Protect from moisture. Do not remove desiccant from the container. Keep bottles tightly closed in a dry place.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise patients:
Diarrhea
- To stop LINZESS and contact their healthcare provider if they experience unusual or severe abdominal pain, and/or severe diarrhea, especially if in combination with hematochezia or melena [see Warnings and Precautions (5.2)].
Accidental Ingestion
- Accidental ingestion of LINZESS in children especially in children less than 6 years of age may result in severe diarrhea and dehydration. Instruct patients to take steps to store LINZESS securely and out of reach of children, and to dispose of unused LINZESS [see Contraindications (4), Warnings and Precautions (5.1, 5.2)].
Administration and Handling Instructions
- To take LINZESS once daily on an empty stomach at least 30 minutes prior to the first meal of the day [see Dosage and Administration (2.2)].
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take 2 doses at the same time.
- To swallow LINZESS capsules whole. Do not crush or chew capsules or capsule contents.
- If adult patients have swallowing difficulties, LINZESS capsules can be opened and administered orally in either applesauce or with bottled water or administered with water via a nasogastric or gastrostomy tube, as described in the Medication Guide.
- To keep LINZESS in the original container. Do not subdivide or repackage. Protect from moisture. Do not remove desiccant from the container. Keep bottles closed tightly in a dry place.
LINZESS® is a registered trademark of Ironwood Pharmaceuticals, Inc.
Distributed by:
Allergan USA, Inc.
Madison, NJ 07940Marketed by:
Allergan USA, Inc. Ironwood Pharmaceuticals, Inc.
Madison, NJ 07940 Cambridge, MA, 02142© 2018 Allergan and Ironwood Pharmaceuticals, Inc. All rights reserved
LINZESS® is a registered trademark of Ironwood Pharmaceuticals, Inc.
Allergan® and its design are trademarks of Allergan, Inc.
Patented. See www.allergan.com/patents.
For more information, go to www.LINZESS.com or call 1-800-678-1605.
-
MEDICATION GUIDE
MEDICATION GUIDE
LINZESS® (lin-ZESS)
(linaclotide)
capsules, for oral useWhat is the most important information I should know about LINZESS?
- Do not give LINZESS to children who are less than 6 years of age. It may harm them.
- You should not give LINZESS to children 6 years to less than 18 years of age. It may harm them.
What is LINZESS?
LINZESS is a prescription medicine used in adults to treat:
- irritable bowel syndrome with constipation (IBS-C).
- a type of constipation called chronic idiopathic constipation (CIC). “Idiopathic” means the cause of the constipation is unknown.
Who should not take LINZESS? -
Do not give LINZESS to children who are less than 6 years of age. LINZESS can cause severe diarrhea and your child could get severe dehydration (loss of a large amount of body water and salt).
- Do not take LINZESS if a doctor has told you that you have a bowel blockage (intestinal obstruction).
Before you take LINZESS, tell your doctor about your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if LINZESS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if LINZESS passes into your breast milk. Talk with your doctor about the best way to feed your baby if you take LINZESS.
How should I take LINZESS?
- Take LINZESS exactly as your doctor tells you to take it.
- Take LINZESS 1 time each day on an empty stomach, at least 30 minutes before your first meal of the day. You should also wait 30 minutes before eating a meal if you take LINZESS with applesauce or mixed with water.
- If you miss a dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time.
- LINZESS capsules should be swallowed whole. Do not crush or chew LINZESS.
- Adults who cannot swallow LINZESS capsules whole may open the LINZESS capsule and sprinkle the LINZESS beads over applesauce or mix LINZESS with bottled water before swallowing.
Taking LINZESS in applesauce:
- Place 1 teaspoon of room temperature applesauce into a clean container. Open the LINZESS capsule and sprinkle all of the LINZESS beads onto the applesauce.
- Swallow all of the LINZESS beads and applesauce right away. Do not keep the applesauce for later use.
- Do not chew the LINZESS beads.
- Pour 1 ounce (30 mL) of room temperature bottled water into a clean cup. Open the LINZESS capsule and sprinkle all of the LINZESS beads into the cup of water.
- Gently swirl the beads and water for at least 20 seconds.
- Swallow all of the LINZESS beads and water mixture right away. Do not keep the mixture for later use.
- If you see any LINZESS beads left in the cup, add another 1 ounce (30 mL) of water to the beads in the cup, swirl for at least 20 seconds, and swallow right away.
Gather the supplies you will need to take your LINZESS dose. Your doctor should tell you what size catheter tipped syringe you will need for your dose. Ask your doctor if you have any questions about how to give LINZESS the right way.- Open the LINZESS capsule and pour all of the LINZESS beads into a clean container with 1 ounce (30 mL) of room temperature bottled water.
- Gently swirl the beads and water for at least 20 seconds.
- Remove the plunger from the catheter tipped syringe, and then pour the LINZESS bead and water mixture into the syringe and replace the plunger.
- Remove the cap from the syringe, insert the tip of the syringe into the nasogastric or gastric feeding tube and push the plunger all the way in to give the dose.
- If you see any LINZESS beads left in the container, add another 1 ounce (30 mL) of water to the beads in the container and repeat the process.
- After giving the LINZESS dose, flush the nasogastric or gastrostomy tube with at least 10 mL of water.
What are the possible side effects of LINZESS?
LINZESS can cause serious side effects, including:
- See “What is the most important information I should know about LINZESS?”
-
Diarrhea is the most common side effect of LINZESS, and it can sometimes be severe.
- Diarrhea often begins within the first 2 weeks of LINZESS treatment.
- Stop taking LINZESS and call your doctor right away if you get severe diarrhea during treatment with LINZESS.
- Diarrhea often begins within the first 2 weeks of LINZESS treatment.
- gas
- stomach-area (abdomen) pain
- swelling, or a feeling of fullness or pressure in your abdomen (distention)
These are not all the possible side effects of LINZESS.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store LINZESS?
- Store LINZESS at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep LINZESS in the bottle that it comes in.
- The LINZESS bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Do not remove the desiccant packet from the bottle.
- Keep the bottle of LINZESS tightly closed and in a dry place.
General information about LINZESS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LINZESS for a condition for which it was not prescribed. Do not give LINZESS to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about LINZESS that is written for health professionals.What are the ingredients in LINZESS?
Active ingredient: linaclotide
Inactive ingredients for the 145 mcg and 290 mcg capsules: calcium chloride dihydrate, hypromellose, L-leucine, and microcrystalline cellulose. Capsule shell: gelatin and titanium dioxide.
Inactive ingredients for the 72 mcg capsules: calcium chloride dihydrate, L-histidine, microcrystalline cellulose, polyvinyl alcohol, and talc. Capsule shell: gelatin and titanium dioxide.
LINZESS® is a registered trademark of Ironwood Pharmaceuticals, Inc.
Distributed by: Allergan USA, Inc. Madison, NJ 07940
Marketed by: Allergan USA, Inc. Madison, NJ 07940 and Ironwood Pharmaceuticals, Inc. Cambridge, MA 02142
© 2018 Allergan and Ironwood Pharmaceuticals, Inc. All rights reserved.
For more information, go to www.LINZESS.com or call 1-800-678-1605.This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 10/2018
- Do not give LINZESS to children who are less than 6 years of age. It may harm them.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LINZESS
linaclotide capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0456-1201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength linaclotide (UNII: N0TXR0XR5X) (linaclotide - UNII:N0TXR0XR5X) linaclotide 145 ug Inactive Ingredients Ingredient Name Strength calcium chloride (UNII: M4I0D6VV5M) Leucine (UNII: GMW67QNF9C) hypromelloses (UNII: 3NXW29V3WO) cellulose, microcrystalline (UNII: OP1R32D61U) titanium dioxide (UNII: 15FIX9V2JP) gelatin (UNII: 2G86QN327L) Product Characteristics Color WHITE (white) Score no score Shape CAPSULE (CAPSULE) Size 16mm Flavor Imprint Code FL;145 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0456-1201-30 1 in 1 CARTON 09/08/2012 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0456-1201-04 1 in 1 CARTON 09/08/2012 2 4 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202811 09/08/2012 LINZESS
linaclotide capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0456-1202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength linaclotide (UNII: N0TXR0XR5X) (linaclotide - UNII:N0TXR0XR5X) linaclotide 290 ug Inactive Ingredients Ingredient Name Strength calcium chloride (UNII: M4I0D6VV5M) Leucine (UNII: GMW67QNF9C) hypromelloses (UNII: 3NXW29V3WO) cellulose, microcrystalline (UNII: OP1R32D61U) titanium dioxide (UNII: 15FIX9V2JP) gelatin (UNII: 2G86QN327L) Product Characteristics Color WHITE (white) Score no score Shape CAPSULE (CAPSULE) Size 18mm Flavor Imprint Code FL;290 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0456-1202-30 1 in 1 CARTON 09/08/2012 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0456-1202-04 1 in 1 CARTON 09/08/2012 2 4 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202811 09/08/2012 LINZESS
linaclotide capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0456-1203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength linaclotide (UNII: N0TXR0XR5X) (linaclotide - UNII:N0TXR0XR5X) linaclotide 72 ug Inactive Ingredients Ingredient Name Strength calcium chloride (UNII: M4I0D6VV5M) histidine (UNII: 4QD397987E) polyvinyl alcohol, unspecified (UNII: 532B59J990) talc (UNII: 7SEV7J4R1U) powdered cellulose (UNII: SMD1X3XO9M) cellulose, microcrystalline (UNII: OP1R32D61U) titanium dioxide (UNII: 15FIX9V2JP) gelatin (UNII: 2G86QN327L) Product Characteristics Color WHITE (white) Score no score Shape CAPSULE (CAPSULE) Size 16mm Flavor Imprint Code FL;72 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0456-1203-30 1 in 1 CARTON 01/30/2017 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0456-1203-04 1 in 1 CARTON 01/30/2017 2 4 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202811 01/30/2017 Labeler - Allergan, Inc. (144796497)
Trademark Results [Linzess]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LINZESS 85773175 4614364 Live/Registered |
Ironwood Pharmaceuticals, Inc. 2012-11-06 |
 LINZESS 77872111 4328588 Live/Registered |
Ironwood Pharmaceuticals, Inc. 2009-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


