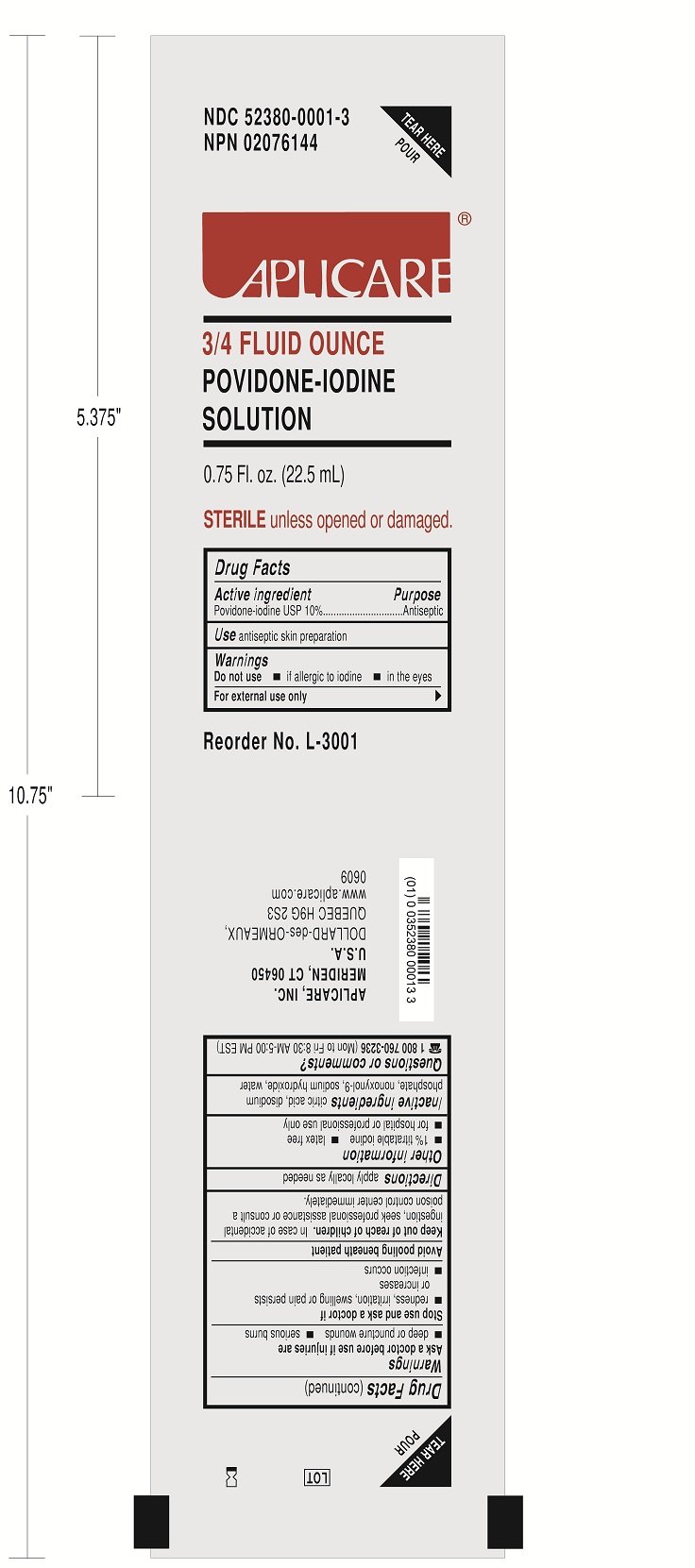
3097-20 CONTINUOUS EPIDURAL 18G HUSTEAD by Smiths Medical ASD, Inc. / Aplicare, Inc.
3097-20 CONTINUOUS EPIDURAL 18G HUSTEAD by
Drug Labeling and Warnings
3097-20 CONTINUOUS EPIDURAL 18G HUSTEAD by is a Other medication manufactured, distributed, or labeled by Smiths Medical ASD, Inc., Aplicare, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
3097-20 CONTINUOUS EPIDURAL 18G HUSTEAD- anesthesia conduction kit
Smiths Medical ASD, Inc.
----------
Do not use
-if allergic to iodine
-in the eyes
For external use only
Ask a doctor before use if injuries are
-deep or puncture wounds
-serious burns
Stop use and ask a doctor if
-redness, irritation, swelling or pain persists or increases
-infection occurs
Avoid pooling beneath patient
Avoid excessive heat. Store at room temperature.
| 3097-20 CONTINUOUS EPIDURAL 18G HUSTEAD
anesthesia conduction kit kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare, Inc. | 081054904 | manufacture | |
Revised: 4/2020
Document Id: 895bbd0e-a445-4d0c-a95e-0e26f7b07b55
Set id: 09f03562-f0ee-4eb1-a7b7-fd0a8a0b51ad
Version: 4
Effective Time: 20200415
Smiths Medical ASD, Inc.