MENATROL- folate, multivitamin capsule
Menatrol by
Drug Labeling and Warnings
Menatrol by is a Prescription medication manufactured, distributed, or labeled by PureTek Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
Full Prescribing Information:
Drug Description:
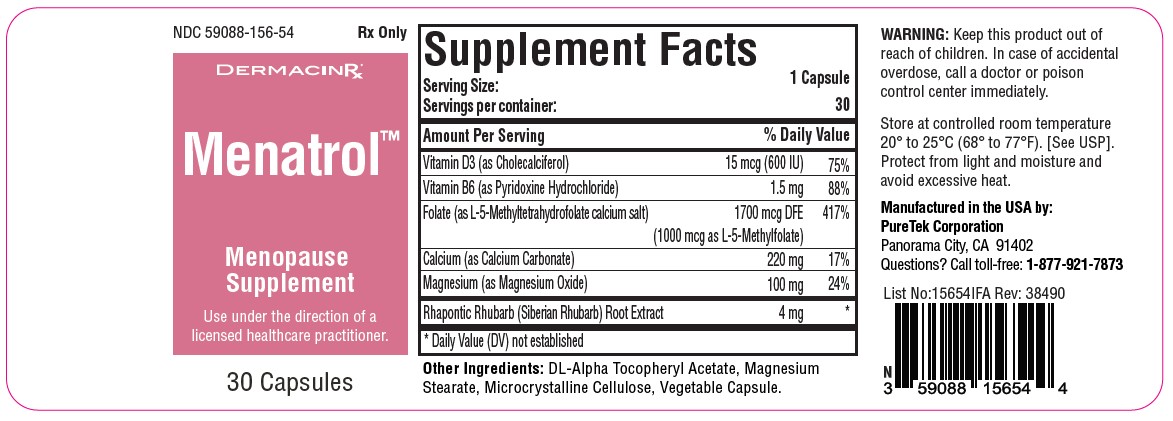
Menatrol™ Capsules are a comprehensive dietary supplement tailored for individuals undergoing menopause. This specialized formulation offers a blend of vitamins, minerals, and plant extracts aiming to alleviate common menopausal symptoms:Vitamin D3 (Cholecalciferol): Contained in a dose of 15 mcg (600 IU), Vitamin D3 plays a pivotal role in maintaining bone health and may also help mitigate mood disturbances. It is recommended to seek a supplement offering 600-800 IU of Vitamin D3 daily, although personal dosing should be discussed with a licensed healthcare practitioner.
Vitamin B6 (Pyridoxine Hydrochloride): At a dosage of 1.5 mg, Vitamin B6 is integral for mood stabilization and promoting energy. Typically, adults are advised to consume 1.3-2.0 mg per day. Always refer to the supplement label for specific dosages.
Folate (L-5-Methyltetrahydrofolate calcium salt): Provided in a measure of 1700 mcg DFE (with 1000 mcg as L-5-Methylfolate), folate is pivotal for holistic health, potentially aiding in mood enhancement and energy regulation. An average adult dosage is between 400-800 mcg daily.
Calcium (Calcium Carbonate): At a concentration of 220 mg, calcium is a key mineral that fortifies bones, particularly crucial during menopause due to heightened osteoporosis risks. Women over 50 typically require around 1000-1300 mg of calcium daily, although intake should be calibrated based on diet.
Magnesium (Magnesium Oxide): Present in an amount of 100 mg, magnesium offers relief from muscle cramps, mood fluctuations, and sleep disturbances. On average, adults are recommended to consume 300-400 mg per day, though individual requirements can differ.
Rhapontic Rhubarb: Rhapontic rhubarb (Rheum rhaponticum) is a natural ingredient that some women use to address menopausal symptoms, including hot flashes and mood swings. The appropriate dosage for this specific ingredient may vary, so consult the product label or a licensed healthcare practitioner for guidance.
Each capsule contains:
Vitamin D3 (as Cholecalciferol)....................................15 mcg (600 IU)
Vitamin B6 (as Pyridoxine Hydrochloride)....................1.5 mg
Folate (as L-5-Methyltetrahydrofolate calcium salt)......1700 mcg DFE (1000 mcg as L-5-Methylfolate)
Calcium (as Calcium Carbonate)..................................220 mg
Magnesium (as Magnesium Oxide)..............................100 mg
Rhapontic Rhubarb (Siberian Rhubarb) Root Extract...4 mg - Other Ingredients:
- Indications and Usage:
- Contraindications:
-
BOXED WARNING
(What is this?)
Warnings:
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor. Administration of folate alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient.
Precautions
Folate in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. There is a potential danger in administering folate to patients with undiagnosed anemia, since folate may obscure the diagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
- Adverse Reactions:
- Drug Interactions:
- Pregnancy and Lactation:
- Overdosage:
- Clinical Pharmacology:
- Nonclinical Toxicology:
- Clinical Studies:
- Dosage and Administration:
-
How Supplied:
Menatrol™Capsules are light yellow powder with black specks, encased in a hard shell capsule. They are packaged in bottle containing 30 capsules – NDC: 59088-156-54. Dispense in a tight, light-resistant container as defined in the USP/NF with a child resistant closure.
- Storage
- Menatrol™
-
INGREDIENTS AND APPEARANCE
MENATROL
folate, multivitamin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59088-156 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 1.5 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 600 [iU] LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 1000 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 100 mg RHUBARB (UNII: G280W4MW6E) (RHUBARB - UNII:G280W4MW6E) RHUBARB 4 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 220 mg Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color yellow (Light Yellow) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59088-156-54 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/18/2023 Labeler - PureTek Corporation (785961046)
Trademark Results [Menatrol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MENATROL 98536552 not registered Live/Pending |
PURETEK CORPORATION 2024-05-06 |
 MENATROL 97939471 not registered Live/Pending |
PURETEK CORPORATION 2023-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.