ALFA VETERINARY 0.9% SODIUM CHLORIDE- sodium chloride, solution irrigant
Alfa Veterinary 0.9% Sodium Chloride by
Drug Labeling and Warnings
Alfa Veterinary 0.9% Sodium Chloride by is a Animal medication manufactured, distributed, or labeled by Laboratorios Alfa SRL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
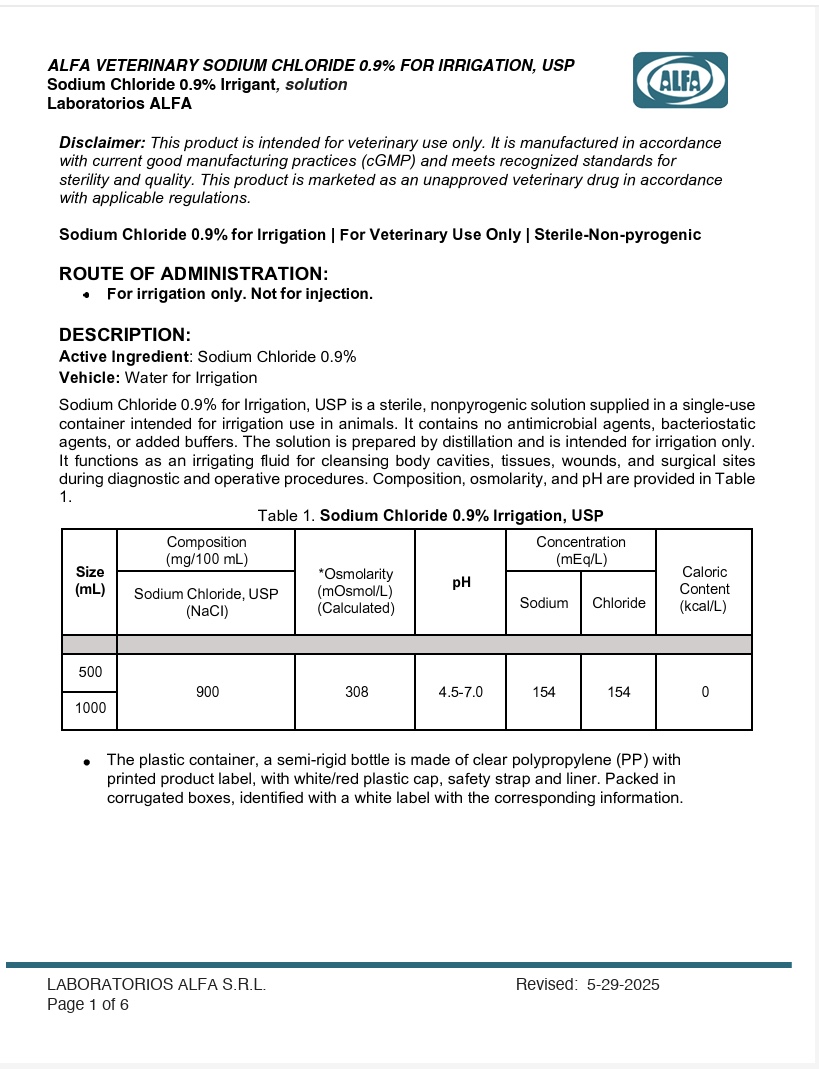
Disclaimer: This product is intended for veterinary use only. It is manufactured in accordance with current good manufacturing practices (cGMP) and meets recognized standards for sterility and quality. This product is marketed as an unapproved veterinary drug in accordance with applicable regulations.
Sodium Chloride 0.9% for Irrigation | For Veterinary Use Only | Sterile-Non-pyrogenic
ROUTE OF ADMINISTRATION: For irrigation only
DESCRIPTION:
Active Ingredient: Sodium Chloride 0.9%
Vehicle: Water for Irrigation
Sodium Chloride 0.9% for Irrigation, USP is a sterile, nonpyrogenic solution supplied in a single-use container intended for irrigation use in animals. It contains no antimicrobial agents, bacteriostatic agents, or added buffers. The solution is prepared by distillation and is intended for irrigation only. It functions as an irrigating fluid for cleansing body cavities, tissues, wounds, and surgical sites during diagnostic and operative procedures. Composition, osmolarity, and pH are provided in Table 1.Table 1.Sodium Chloride for Irrigation 0.9% Irrigation, USP
Size (mL) Composition (mg/100 mL) *Osmolarity (mOsmol/L)(Calculated) pH Concentration (mEq/L) Caloric Content (kcal/L) Sdoium Chloride, USP (Nacl) Sodium Chloride 500 900 308 4.5-7.0 154 154 0 1000 The plastic container, a semi-rigid bottle is made of clear polypropylene (PP) with printed product label, with white/red plastic cap, safety strap and liner. Packed in corrugated boxes, identified with a white label with the corresponding information.
-
CLINICAL PHARMACOLOGY (Veterinary Use):
Sodium Chloride 0.9% Irrigation, USP is sterile, nonpyrogenic and isotonic solution with an osmolarity of approximately 308 mOsmol/L. It is physiologically compatible with animal tissues and is intended for irrigation of body cavities, tissues, and surgical sites. The solution contains no antimicrobial agents or preservatives.
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
WARNINGS:
For irrigation only. Not for injection or parenteral administration.
See Instructions for Use for inspection guidelines.
Large volumes of irrigation fluid may be absorbed systemically, which can lead to fluid and/or solute overload, dilution of serum electrolytes, overhydration, congested states, or pulmonary edema
When used as a diluent, verify compatibility with additives
The risk of dilutional states is inversely proportional to electrolyte concentration; the risk of solute overload is directly proportional to electrolyte concentration
Avoid use during electrosurgical procedures due to potential electrical conduction risks
Single-use container. Discard any unused portion promptly after opening to minimize the risk of contamination or pyrogen formation.The container label bears the statement:
See package insert for complete warnings
Keep out of the reach of children -
PRECAUTIONS:
Inspect solution before use. See Instructions for Use.
Use with caution in animals with cardiac or renal impairment, or in those predisposed to fluid overload, as systemic absorption of irrigation fluid may occur
Monitor for signs of overhydration, electrolyte imbalance, or congestive states, especially when large volumes are used or in small or compromised animals
Do not use if the product is expired, the lot number is missing or illegible
Maintain aseptic conditionsUSE IN PREGNANT OR LACTATING ANIMALS:
The safety of Sodium Chloride 0.9% for Irrigation, USP has not been established in pregnant or lactating animals. Use only when the potential benefits justify the potential risks to the fetus or neonate. Veterinarian discretion is advised. A documented risk-benefit assessment is recommended.
ADVERSE EVENT REPORTING
To report suspected adverse reactions, contact Vedco Inc. at (888) 708-3326 or report to the FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae. -
ADVERSE REACTIONS:
If an adverse reaction occurs, discontinue irrigation immediately, evaluate the animal, and initiate appropriate veterinary treatment. Retain the remainder of the solution for examination if necessary. Reported adverse reactions include: · Febrile response, · Infection at the site of irrigation, · Local irritation, · Extravasation, · Systemic effects such as hypervolemia if large volumes are absorbed
- OVERDOSAGE:
-
DOSAGE AND ADMINISTRATION:
This is a single-use container. See Instructions for Use for inspection guidelines.
Use only as directed by a veterinarian. The volume used depends on the size of the animal, the area to be irrigated, and the clinical situation.
Avoid excessive pressure during irrigation to prevent tissue damage and minimize systemic absorption
Aseptic technique must be used throughout handling and administration.
If additives are introduced, use aseptic technique and mix thoroughly
Do not store any solution containing additives. See Shelf Life for disposal instructions. -
HOW SUPPLIED:
Veterinary Sodium Chloride 0.9% for Irrigation, USP solution is available in the following sizes:
BOTTLES NDC#
Sodium chloride 0.9% for Irrigation, 500 mL NDC: 72483-207-01
Sodium chloride 0.9% for Irrigation,1000 mL NDC 72483-207-02
-
INSTRUCTIONS FOR USE:
PLASTIC BOTTLE
NOTE: Before use, perform the following checks:Read the label. Verify the solution composition, lot number, and expiration date on the container.
Do not use if the product is expired, the lot number is missing or illegible, or if the solution is cloudy or contains a precipitate.
Invert the container and inspect in good light for leaks or particulate matter. If leaks are found or the seal is not intact, discard the solution.
Use only if the solution is clear and the container and seal are intact.Preparation for Administration:
1. Use aseptic technique when preparing and handling the container.
2. Outer Closure Removal – Grasp the container with one hand and turn the breakaway ring counterclockwise with the other hand until slight resistance is felt.
3. Then, twisting the container in the opposite direction, turn the breakaway ring sharply until the entire outer cap is loose and can be lifted off.
4. Remove screw cap and pour.
5. Once opened, use immediately and discard any unused portion.INTRODUCTION OF ADDITIVES:
Warning: Some additives may be incompatible.Known incompatible additives include:
Strong oxidizing agents
Alcohol-containing solutions
Solutions with high electrolyte concentrationsAlways verify compatibility before mixing. If incompatibility is suspected, do not use the mixture.
To Add Medication Before Solution Administration.
1. If additives are introduced, follow aseptic technique as described above.
2. Mix solution and medication thoroughly before use. -
STORAGE:
Store at a temperature not higher than 30ºC (86ºF). Avoid excessive heat. Protect from freezing.
CAUTION: Federal law restricts this drug (USA) for use by or on the order of a licensed veterinarian.
Manufactured by:
LABORATORIOS ALFA S.R.L
Santo Domingo, Dominican Republic
www.laboratoriosalfa.com
Toll Free: +1-800-969-0581
Made in Dominican Republic - PACKAGE INSERT
-
0.9% SODIUM CHLORIDE 0.9%, FOR IRRIGATION USP
For Veterinary Use Only
Sodium chloride 0.9% for Irrigation, 500 mL NDC: 72483-207-01
Sodium chloride 0.9% for Irrigation,1000 mL NDC: 72483-207-02


-
INGREDIENTS AND APPEARANCE
ALFA VETERINARY 0.9% SODIUM CHLORIDE
sodium chloride, solution irrigantProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 72483-207 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72483-207-02 1000 mL in 1 BOTTLE, PLASTIC 2 NDC: 72483-207-01 500 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/15/2025 Labeler - Laboratorios Alfa SRL (815941244)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.