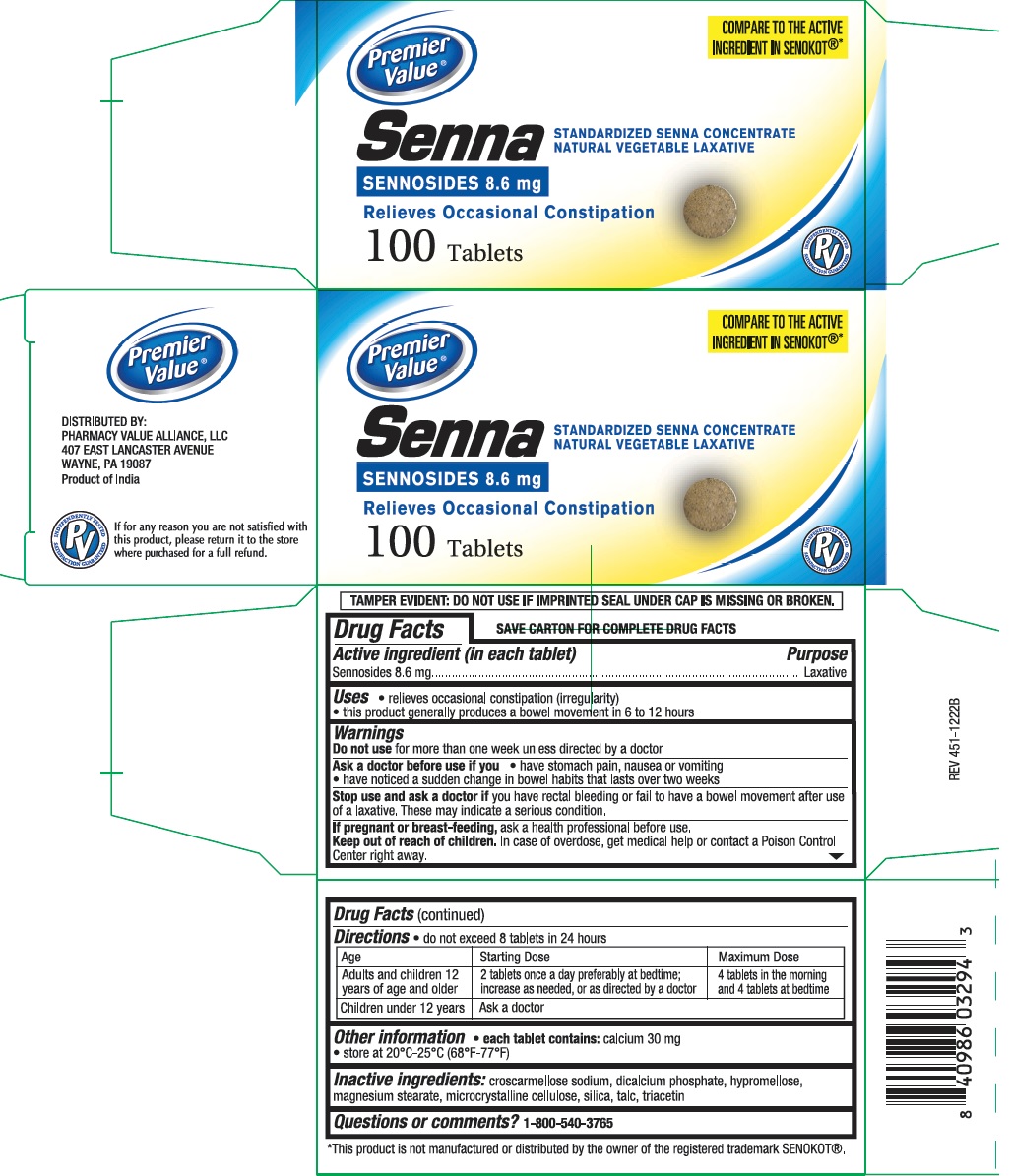
Senna by PHARMACY VALUE ALLIANCE LLC / Geri-Care Pharmaceutical Corp pva 451B
Senna by
Drug Labeling and Warnings
Senna by is a Otc medication manufactured, distributed, or labeled by PHARMACY VALUE ALLIANCE LLC, Geri-Care Pharmaceutical Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SENNA- sennosides tablet
PHARMACY VALUE ALLIANCE LLC
----------
pva 451B
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
for more than one week unless directed by a doctor.
Ask a doctor before use if you
have stomach pain, nausea or vomiting
have noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if
you have rectal bleeding or fail to have a bowel movement after use of a laxative.
These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
do not exceed 8 tablets in 24 hours
| Age
| Starting Dose
| Maximum Dose
|
|---|---|---|
| adults and children 12 years of age and older
| 2 tablets once a day preferably at bedtime; increase as needed, or as directed by a doctor
| 4 tablets in the morning and 4 tablets at bedtime
|
| children under 12 years
| ask a doctor
|
Other information
- each tablet contains: calcium 30 mg
- Tamper Evident: Do not use if imprinted seal under cap is missing or broken
- store at 20°C-25°C (68°F-77°F)
| SENNA
sennosides tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PHARMACY VALUE ALLIANCE LLC (101668460) |
| Registrant - Geri-Care Pharmaceutical Corp (611196254) |
Revised: 11/2024
Document Id: 27feafe1-abde-8ed2-e063-6394a90aaa68
Set id: 0a3502f3-f83d-0417-e063-6294a90a88a1
Version: 3
Effective Time: 20241128
Trademark Results [Senna]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENNA 97868919 not registered Live/Pending |
Natty Collection LLC 2023-04-02 |
 SENNA 90583748 not registered Live/Pending |
Jeremey Decena 2021-03-17 |
 SENNA 90399285 not registered Live/Pending |
AYRTON SENNA EMPREENDIMENTOS LTDA. 2020-12-21 |
 SENNA 90022160 not registered Live/Pending |
OPWEST DEVELOPMENT LLC 2020-06-26 |
 SENNA 88624114 not registered Live/Pending |
Ceritas Wines LLC 2019-09-19 |
 SENNA 87683504 5564030 Live/Registered |
OMM Imports Inc. 2017-11-14 |
 SENNA 76601884 3268781 Dead/Cancelled |
Studio RTA 2004-07-12 |
 SENNA 75170094 2188775 Live/Registered |
Senna Cosmetics, Inc. 1996-09-23 |
 SENNA 74561186 not registered Dead/Abandoned |
Senna Cosmetics, Inc. 1994-08-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.