SUNSCREEN SPF30- avobenzone, homosalate, octisalate, octocrylene lotion
Sunscreen SPF30 by
Drug Labeling and Warnings
Sunscreen SPF30 by is a Otc medication manufactured, distributed, or labeled by Broder Bros Prime Line. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
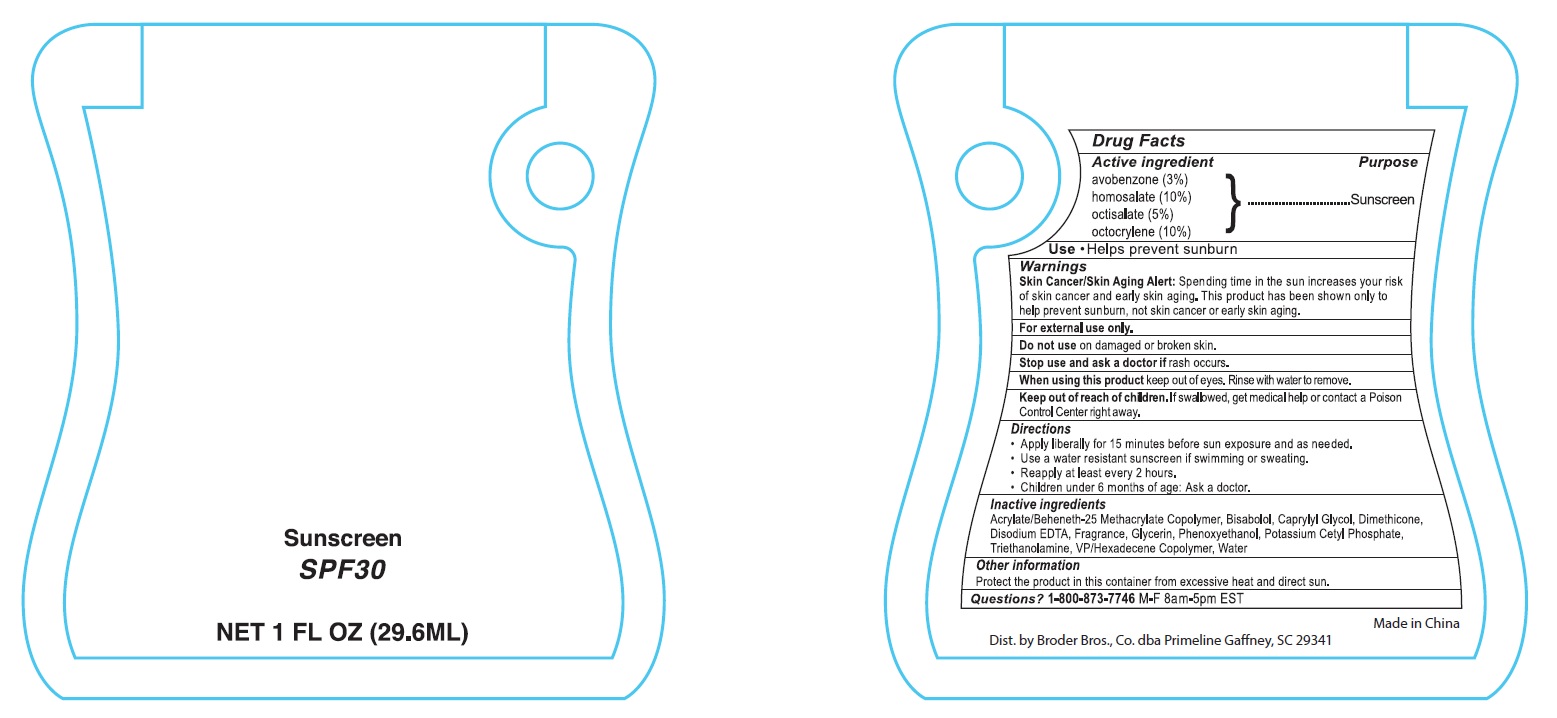
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- Other information
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUNSCREEN SPF30
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71513-708 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength LEVOMENOL (UNII: 24WE03BX2T) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) TROLAMINE (UNII: 9O3K93S3TK) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71513-708-01 29.6 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/09/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/09/2025 Labeler - Broder Bros Prime Line (107044246)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.