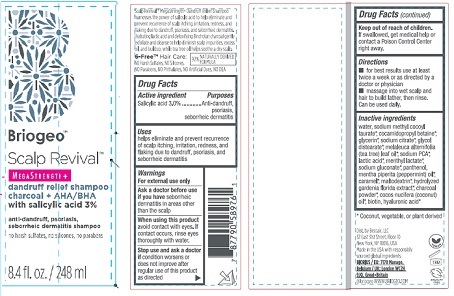
SCALP REVIVAL- salicylic acid 3% shampoo
Scalp Revival by
Drug Labeling and Warnings
Scalp Revival by is a Otc medication manufactured, distributed, or labeled by Beccair, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Purposes
- Uses
-
Warnings
For external use only
Ask a doctor before use
if you have seborrhic
dermatitis in areas other
than the scalp
When using this product
avoid contact with eyes. If
contact occurs, rinse eyes
thoroughly with water.
Stop use and ask a doctor
if condition worsens or
does not improve after
regular use of tjis product
as directed
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
water, sodium methyl cocoyl
taurate*, cocamidopropyl betaine*,
glycerin*, sodium citrate*, glycol
distearate*, melaleuca alternifolia
(tea tree) leaf oil*, sodium PCA*,
lactic acid*, menthyl lactate*,
sodium gluconate*, panthenol,
mentha piperita (peppermint) oil*,
caramel*, maltodextrin*, hydrolyzed
gardenia florida extract*, charcoal
powder*, cocos nucifera (coconut)
oil*, biotin, hyaluronic acid*
* Coconut, vegetable, or plant derived
- Product Label
-
INGREDIENTS AND APPEARANCE
SCALP REVIVAL
salicylic acid 3% shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82594-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 3.0 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CITRATE (UNII: 1Q73Q2JULR) GLYCOL DISTEARATE (UNII: 13W7MDN21W) TEA TREE OIL (UNII: VIF565UC2G) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) LACTIC ACID (UNII: 33X04XA5AT) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) SODIUM GLUCONATE (UNII: R6Q3791S76) PANTHENOL (UNII: WV9CM0O67Z) PEPPERMINT OIL (UNII: AV092KU4JH) CARAMEL (UNII: T9D99G2B1R) MALTODEXTRIN (UNII: 7CVR7L4A2D) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) COCONUT OIL (UNII: Q9L0O73W7L) BIOTIN (UNII: 6SO6U10H04) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82594-001-50 248 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/28/2022 2 NDC: 82594-001-51 11.8 mL in 1 PACKET; Type 0: Not a Combination Product 02/28/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/28/2022 Labeler - Beccair, LLC (078809020)
Trademark Results [Scalp Revival]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SCALP REVIVAL 98196646 not registered Live/Pending |
Beccair LLC 2023-09-25 |
 SCALP REVIVAL 97548050 not registered Live/Pending |
Beccair LLC 2022-08-14 |
 SCALP REVIVAL 87254119 5361182 Live/Registered |
Beccair, LLC 2016-12-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.