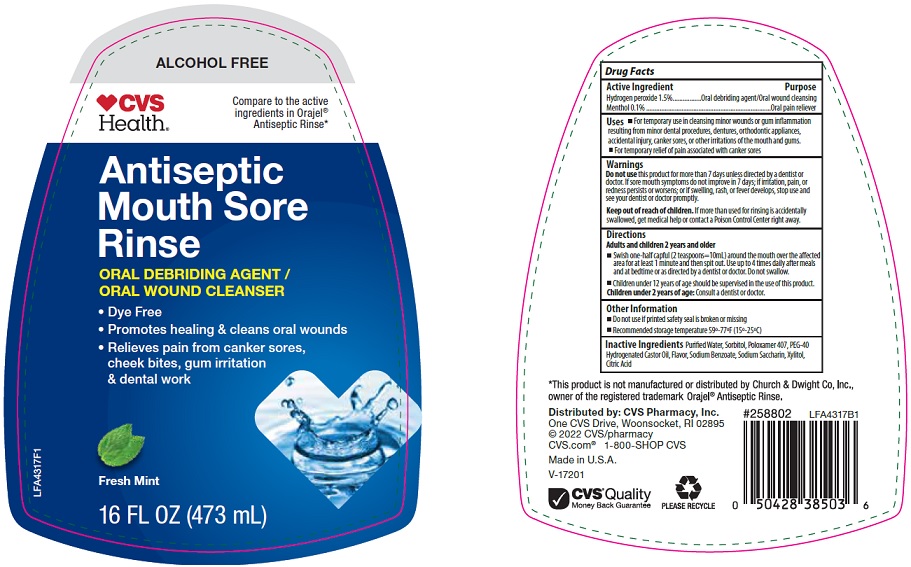
CVS Pharmacy, Inc. Antiseptic Mouth Sore Rinse Drug Facts
Antiseptic Mouth Sore by
Drug Labeling and Warnings
Antiseptic Mouth Sore by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTISEPTIC MOUTH SORE- hydrogen peroxide, menthol rinse
CVS Pharmacy
----------
CVS Pharmacy, Inc. Antiseptic Mouth Sore Rinse Drug Facts
Uses
- For temporary use in cleansing minor wounds or gum inflammation resulting from minor dental procedures, dentures, orthodontic appliances, accidental injury, canker sores, or other irritations of the mouth and gums.
- For temporary relief of pain associated with canker sores
Warnings
Directions
Adults and children 2 years and older
- Swish one-half capful (2 teaspoons=10mL) around the mouth over the affected area for at least 1 minute and then spit out. Use up to 4 times daily after meals and at bedtime or as directed by a dentist or doctor. Do not swallow.
- Children under 12 years of age should be supervised in the use of this product.
Children under 2 years of age: Consult a dentist or doctor.
Other Information
- Do not use if printed safety seal is broken or missing
- Recommended storage temperature 59°-77°F (15°-25°C)
Inactive Ingredients
Purified Water, Sorbitol, Poloxamer 407, PEG-40 Hydrogenated Castor Oil, Flavor, Sodium Benzoate, Sodium Saccharin, Xylitol, Citric Acid
Package/Label Principal Display Panel
ALCOHOL FREE
CVS Health®
Compare to the active ingredients in Orajel® Antiseptic Rinse
Antiseptic Mouth Sore Rinse
ORAL DEBRIDING AGENT / ORAL WOUND CLEANSER
- Dye Free
- Promotes healing & cleans oral wounds
- Relieves pain from canker sores, cheek bites, gum irritation & dental work
Fresh Mint
16 FL OZ (473 mL)
LFA4317F1
| ANTISEPTIC MOUTH SORE
hydrogen peroxide, menthol rinse |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.