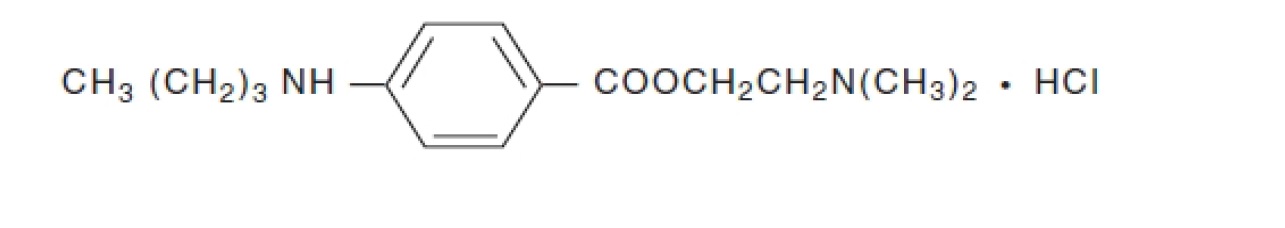TETRACAINE HYDROCHLORIDE solution/ drops
Tetracaine Hydrochloride by
Drug Labeling and Warnings
Tetracaine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Paragon BioTeck, Inc., Bausch & Lomb Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% safely and effectively. See full prescribing information for Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5%.
Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5%, for topical
ophthalmic use
Initial U.S. Approval: 1965INDICATIONS AND USAGE
Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5%, is an ester local anesthetic indicated for procedures requiring a rapid and short-acting topical ophthalmic anesthetic. (1)
DOSAGE AND ADMINISTRATION
One drop topically in the eye(s) as needed. (2)
DOSAGE FORMS AND STRENGTHS
Sterile, preserved, ophthalmic solution containing 0.5% tetracaine hydrochloride. (3)
CONTRAINDICATIONS
Tetracaine Hydrochloride Ophthalmic Solution, 0.5% should not be used in patients with a history of hypersensitivity to any component of this preparation. (4)
WARNINGS AND PRECAUTIONS
- Do not use intracamerally since use may damage corneal endothelial cells. (5.1)
- Prolonged use or abuse may lead to corneal epithelial toxicity and may manifest as epithelial defects which may progress to permanent corneal damage. (5.2)
- Patients should not touch the eye for at least 10-20 minutes after using anesthetic as accidental injuries can occur due to insensitivity of the eye. (5.3)
ADVERSE REACTIONS
Ocular adverse events: transient stinging, burning, conjunctival redness, eye irritation, eye pain, ocular discomfort. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb, a Division of Valeant Pharmaceuticals North America LLC,. at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Corneal Injury with Intracameral Use
5.2 Corneal Toxicity
5.3 Corneal Injury due to Insensitivity
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Corneal Injury with Intracameral Use
Not for injection or intraocular use. Do not use intracamerally because use of Tetracaine
Hydrochloride Ophthalmic Solution USP, 0.5% may lead to damage of the corneal endothelial
cells.
-
6 ADVERSE REACTIONS
The following serious ocular adverse reactions are described elsewhere in the labeling:
- Corneal Injury with Intracameral Use [See Warnings and Precautions (5.1)]
- Corneal Toxicity [See Warnings and Precautions (5.2)]
- Corneal Injury due to Insensitivity [See Warnings and Precautions (5.3)]
The following adverse reactions have been identified following use of Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliable estimate their frequency or establish a causal relationship to drug exposure.
Ocular Adverse Reactions
Transient stinging, burning, and conjunctival redness, eye irritation, eye pain, ocular discomfort.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with Tetracaine Hydrochloride Ophthalmic
Solution USP, 0.5% in pregnant women. Animal developmental and reproductive toxicity studies
with tetracaine hydrochloride have not been reported in the published literature.
8.2 Lactation
Risk Summary
There are no data to assess whether Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% is
excreted in human milk or to assess its effects on milk production/excretion. The developmental
and health benefits of breastfeeding should be considered along with the mother’s clinical need
for Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% and any potential adverse effects
on the breastfed child from Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5%.
8.3 Females and Males of Reproductive Potential
No human data on the effect of Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% on
fertility are available.
8.4 Pediatric Use
Safety of Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% in the pediatric population
has been demonstrated in clinical trials. Efficacy of Tetracaine Hydrochloride Ophthalmic
Solution USP, 0.5% for use in pediatric patients has been extrapolated from adequate and well
controlled clinical trials in the adult population.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% is a sterile, clear, colorless, topical
local anesthetic for ophthalmic use containing tetracaine hydrochloride as the active
pharmaceutical ingredient.
Tetracaine hydrochloride is chemically designated as benzoic acid, 4-(butylamino)-,
2-(dimethylamino) ethyl ester, monohydrochloride. Its chemical formula is C15H24N2O2
HCl and it is represented by the chemical structure:
Tetracaine hydrochloride is a fine, white, crystalline, odorless powder with a molecular weight of
300.82
Active ingredient: tetracaine hydrochloride 0.5% w/v (equivalent to 0.44% w/v tetracaine)
Preservative: chlorobutanol 0.4%
Inactive ingredients: boric acid, potassium chloride, edetate disodium, water for injection USP.
Sodium hydroxide and/or hydrochloric acid may be added to adjust pH (3.7 – 6.0)
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the genotoxicity of tetracaine hydrochloride have not been reported in the
published literature. Long-term animal studies have not been conducted to evaluate the
carcinogenic potential of tetracaine hydrochloride. Animal studies to assess the effects of
tetracaine hydrochloride on fertility have not been reported in the published literature.
-
14 CLINICAL STUDIES
Topical administration of Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% results in
localized temporary anesthesia. The maximum effect is achieved within 10–20 seconds after
instillation, with efficacy lasting 10–20 minutes. Duration of effect can be extended with
repeated dosing. [See Warnings and Precautions (5.2) and Overdosage (10)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tetracaine Hydrochloride Ophthalmic Solution USP, 0.5% is supplied as a sterile, aqueous,
topical ophthalmic solution with a fill volume of 15 mL in a 15 mL low-density polyethylene
plastic dropper bottle with a low-density polyethylene dropper tip and white polypropylene cap.
NDC: 42702-170-15
After opening, this product can be used until the expiration date stamped on the bottle.
Storage: Store at 15°C to 25°C (59°F to 77°F). Protect from light. Do not use if solution
contains crystals, cloudy, or discolored.
-
17 PATIENT COUNSELING INFORMATION
Eye Care Precaution
Do not touch the dropper tip to any surface as this may contaminate the solution.
Advise patients that, due to the effect of the anesthetic, their eyes will be insensitive for up to 20
minutes and that care should be taken to avoid accidental injuries.
Manufactured for:
Paragon BioTeck, Inc.
4640 SW Macadam Ave, Ste 80
Portland, OR 97239
Manufactured and Distributed by:
Bausch & Lomb
8500 Hidden River Pkwy
Tampa, FL 33637
Revised: March 2019
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- Carton 15 mL
-
INGREDIENTS AND APPEARANCE
TETRACAINE HYDROCHLORIDE
tetracaine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42702-170 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACAINE HYDROCHLORIDE (UNII: 5NF5D4OPCI) (TETRACAINE - UNII:0619F35CGV) TETRACAINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength CHLOROBUTANOL (UNII: HM4YQM8WRC) BORIC ACID (UNII: R57ZHV85D4) POTASSIUM CHLORIDE (UNII: 660YQ98I10) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42702-170-15 1 in 1 CARTON 03/12/2019 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210821 03/12/2019 Labeler - Paragon BioTeck, Inc. (078279037) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(42702-170)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.