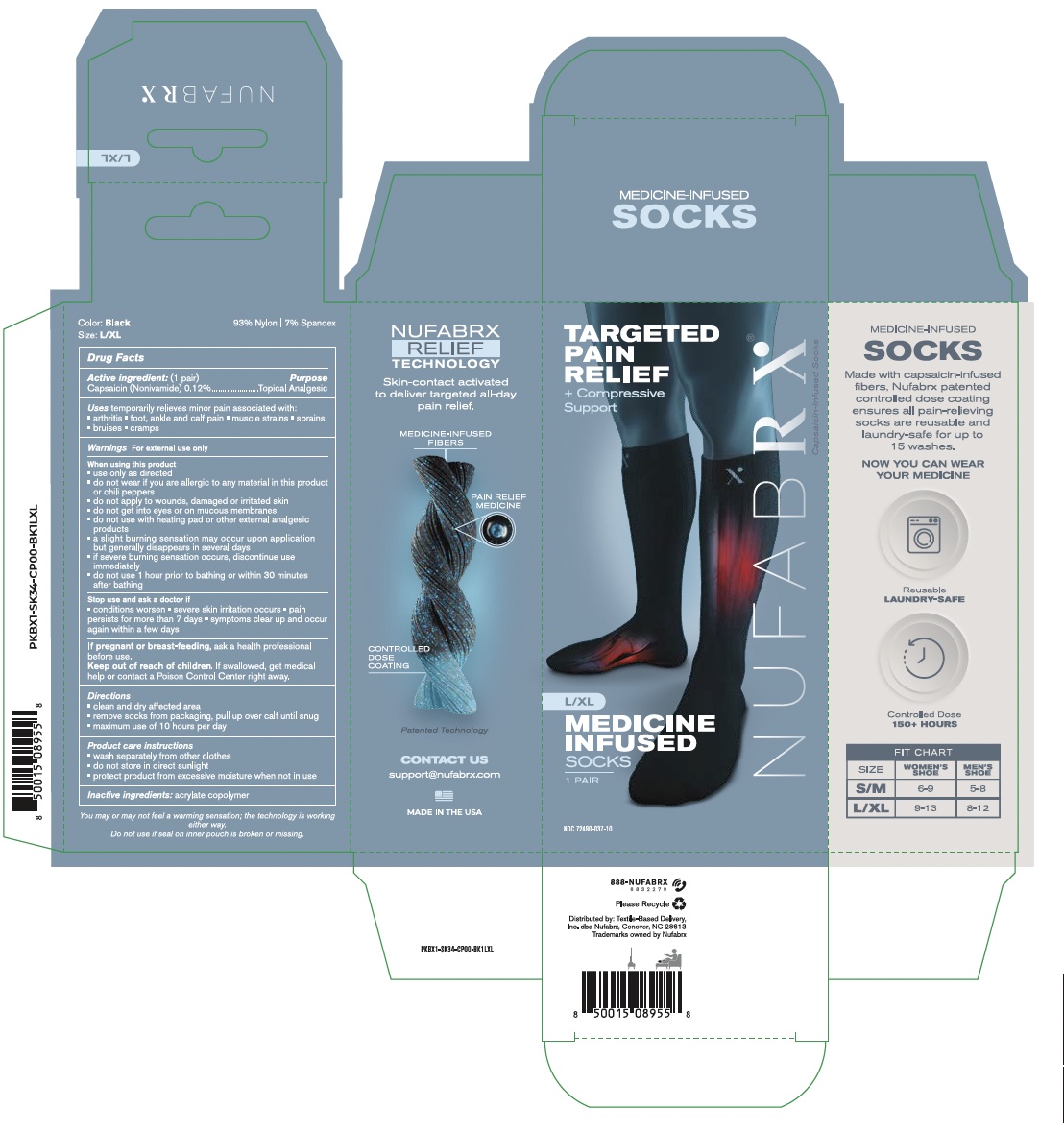
Rx Clothing Pain Relief Socks 72490-100
Nufabrx Rx Clothing Pain Relief Socks by
Drug Labeling and Warnings
Nufabrx Rx Clothing Pain Relief Socks by is a Otc medication manufactured, distributed, or labeled by Nufabrx LLC, Biotech Research Group, Textile-Based Delivery Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NUFABRX RX CLOTHING PAIN RELIEF SOCKS- capsaicin cloth
Nufabrx LLC
----------
Rx Clothing Pain Relief Socks 72490-100
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with: Simple backache, arthritis, strains, bruises and sprains.
Warnings
For external use only
Do not use
-If you are allergic to any ingredients of this product or chili peppers, contact a doctor before use (allergy alert)
-On wounds or damaged skin
-With, or at the same time as, other external analgesic products
-With a heating pad
When using this product
-You may experience a burning sensation which is normal and related to the way the product works (with regular use, this sensation generally diminishes)
-Do not use immediately before or after activities such as swimming, showering, sunbathing or intense exercise
-Use only as directed
-Avoid contact with eyes and mucous membranes or rashes
Directions
-Clean and dry affected area
-Remove socks from packaging and place one sock on each foot
-Maximum use of 10 hours per day
-Wash separately from other clothing
-Do not wear more than 6 days straight without doctor consent
| NUFABRX RX CLOTHING PAIN RELIEF SOCKS
capsaicin cloth |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Nufabrx LLC (119110776) |
| Registrant - Biotech Research Group (099152813) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Textile-Based Delivery Inc | 079309716 | api manufacture(72490-100) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.