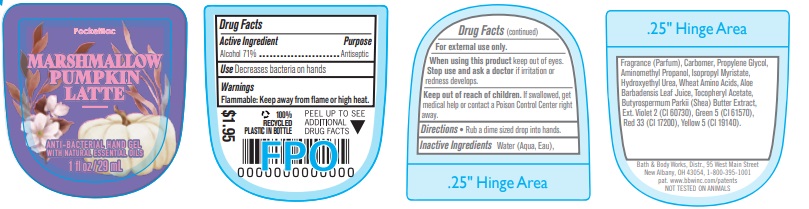
Active Ingredient
Alcohol 71%
Use
Decreases bacteria on hands.
Warnings
Flammable: Keep away from flame or high heat.
When using this product keep out of eyes. Stop use and ask a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Rub a dime sized drop into hands.
INACTIVE INGREDIENTS: Water (Aqua, Eau), Fragrance (Parfum), Carbomer, Propylene Glycol, Aminomethyl Propanol, Isopropyl Myristate, Hydroxyethyl Urea, Wheat Amino Acids, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Butyrospermum Parkii (Shea) Butter Extract, Ext. Violet 2 (CI 60730), Green 5 (CI 61570), Red 33 (CI 17200), Yellow 5 (CI 19140).
Bath & Body Works, Distr., 95 West Main Street
New Albany, OH 43054, 1-800-395-1001
pat. www.bbwinc.com/patents