KIT FOR THE PREPARATION OF TECHNETIUM TC99M MERTIATIDE- betiatide injection, powder, lyophilized, for solution
Kit for the Preparation of Technetium Tc99m Mertiatide by
Drug Labeling and Warnings
Kit for the Preparation of Technetium Tc99m Mertiatide by is a Prescription medication manufactured, distributed, or labeled by Sun Pharmaceutical Industries, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
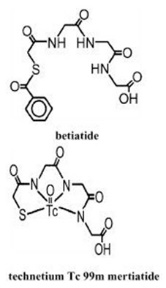
Kit for the Preparation of Technetium Tc99m Mertiatide is used for the preparation of technetium Tc 99m mertiatide, a diagnostic radiopharmaceutical. It is supplied as a sterile, nonpyrogenic, lyophilized powder. Each vial contains betiatide (N-[N-[N-[(benzoylthio) acetyl]glycyl]glycyl]-glycine). After reconstitution with sterile sodium pertechnetate Tc 99m injection, the technetium Tc 99m mertiatide (disodium[N-[N-[N-(mercaptoacetyl) glycyl]glycyl] glycinato (2-) - N,N′,N″,S′]oxotechnetate (2-)) which is formed is suitable for intravenous administration.
Each 10 milliliter vial contains 1 milligram betiatide, 0.05 milligram (minimum) stannous chloride dihydrate (SnCl2∙2H2O) and 0.2 milligram (maximum) total tin expressed as stannous chloride dihydrate (SnCl2∙2H2O), 40 milligrams sodium tartrate dihydrate (Na2C4H2O6∙2H2O), and 20 milligrams lactose monohydrate. Prior to lyophilization, sodium hydroxide or hydrochloric acid may be added for pH adjustment. The pH of the reconstituted drug is between 5.0 and 6.0. No bacteriostatic preservative is present. The contents are sealed under argon. Betiatide is light sensitive and must be protected from light. Betiatide and technetium Tc 99m mertiatide have the following structural formulas:
-
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging is listed in Table 1.
Table 1: Principal Radiation Emission Data* Radiation
MMean % per Disintegration Energy
(keV)- * Kocher, David C., "Radioactive Decay Tables," DOE/TIC-11026, 108 (1981).
Gamma-2
89.07
140.5
The specific gamma ray constant for Technetium Tc 99m is 0.78 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for Technetium Tc 99m is 0.017 cm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.25 cm of Pb will decrease the external radiation exposure by a factor of about 1000.
Table 2: Radiation Attenuation by Lead Shielding Shield Thickness
(Pb) cmCoefficient of Attenuation 0.017
0.5
0.08
10-1
0.16
10-2
0.25
10-3
0.33
10-4
To correct for physical decay of the radionuclide, the fractions that remain at selected time intervals after the time of calibration are shown in Table 3.
-
CLINICAL PHARMACOLOGY
Following intravenous injection of technetium Tc 99m mertiatide, the appearance, concentration, and excretion of the tracer in the kidney can be monitored to assess renal function. Although technetium Tc 99m mertiatide is highly plasma protein bound following intravenous injection, the protein binding is reversible and the tracer is rapidly excreted by the kidneys via active tubular secretion and glomerular filtration. Following intravenous injection of technetium Tc 99m mertiatide in normal volunteers, 89% of the tracer was plasma protein bound. In healthy subjects with normal renal function (mean serum creatinine 1.2 mg/dL) technetium Tc 99m mertiatide was rapidly cleared from the blood. The plasma clearance was approximately 0.3 liters/minute and the amount of technetium Tc 99m mertiatide excreted in the urine in three hours was nearly 90% of the dose. In a study performed in three patients with renal impairment (serum creatinine greater than 6.3 mg/dL), there was decreased blood clearance and a decrease in the amount excreted in the urine over three hours. In these patients, 78% of the tracer was plasma protein bound after intravenous injection. The mean plasma clearance of technetium Tc 99m mertiatide was 0.03 liters/minute and 21.3% was excreted in three hours on average. In both healthy subjects and patients with renal impairment, the plasma concentration-time profile showed a biexponential decline.
-
INDICATIONS AND USAGE
Technetium Tc 99m mertiatide is a renal imaging agent for use in the diagnosis of congenital and acquired abnormalities, renal failure, urinary tract obstruction, and calculi in adults and pediatric patients. (See Pediatric Use.) It is a diagnostic aid in providing renal function, split function, renal angiograms, and renogram curves for whole kidney and renal cortex.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
1. The contents of this kit are not radioactive. However, after sodium pertechnetate Tc 99m is added, adequate shielding of the final preparation must be maintained.
2. Contents of the reaction vial are intended only for use in the preparation of technetium Tc 99m mertiatide and are NOT to be administered directly to the patient.
3. To help reduce the radiation dose to the bladder, as well as other target organs, the patient should increase his or her fluid intake (unless medically contraindicated) and void as often as possible after the injection of technetium Tc 99m mertiatide for six hours after the imaging procedure.
4. Technetium Tc 99m mertiatide should not be used more than six hours after preparation.
5. The components of the kit are sterile and nonpyrogenic. It is essential that the user follow the directions carefully and use aseptic procedures normally employed in making additions and withdrawals from sterile, nonpyrogenic containers during the addition of pertechnetate solution and the withdrawal of doses for patient administration.
6. The technetium Tc 99m labeling reactions involved in preparing technetium Tc 99m mertiatide depend on maintaining the stannous ion in the reduced state. Any oxidant present in the sodium pertechnetate Tc 99m may adversely affect the quality of the radiopharmaceutical. Therefore, sodium pertechnetate Tc 99m containing oxidants should not be employed.
7. As in the use of any other radioactive material, care should be taken to insure minimum radiation exposure to the patient and to occupational workers.
8. Radiopharmaceuticals should be used only by physicians who are qualified by specific training in the safe use and handling of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term animal studies have been performed to evaluate carcinogenic or mutagenic potential, or whether this drug affects fertility in males or females.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with technetium Tc 99m mertiatide. It is also not known whether this drug can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Technetium Tc 99m mertiatide should be given to a pregnant woman only if clearly needed.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The suggested dose range employed in the average adult patient (70kg) for renal function and imaging studies is 185 MBq (5 mCi) to 370 MBq (10 mCi). In pediatric patients the recommended dose range is 2.6 MBq/kg (70 μCi/kg) to 5.2 MBq/kg (140 μCi/kg) with a minimum dose of 37 MBq (1 mCi).
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Aseptic procedures and a shielded syringe should be employed in withdrawing doses for administration to patients. The user should wear waterproof gloves during the administration procedure.
-
RADIATION DOSIMETRY
The estimated absorbed radiation doses from an intravenous administration of technetium Tc 99m mertiatide are presented in Table 4.
Table 4: Radiation Dose Estimates for Tc 99m Mertiatide 8-day Old 1-year Old* 5-year Old* 10-year Old* 15-year Old Adult Assumed Weight (kg) 3.4 9.8 19 32 57 70 Tc 99m Mertiatide Dose 37 MBq
(1 mCi)72.52 MBq
(1.96 mCi)140.6 MBq
(3.8 mCi)236.8 MBq
(6.4 mCi)370 MBq
(10 mCi)370 MBq
(10 mCi)Organ mSv rem mSv rem mSv rem mSv rem mSv rem mSv rem *Calculated by Oak Ridge Associated Universities, based upon the pediatric phantom series of Christy and Eckerman of Oak Ridge National Laboratories. The adult radiation absorbed doses were calculated based on data from ten normal volunteers using the Medical Internal Radiation Dose Committee (MIRD) schema. - * Radioactive doses for 1-, 5-, and 10-year olds are based on a maximum dose of 7.4 MBq/kg (200 μCi/kg).
Gallbladder Wall
2.701
0.27
2.466
0.235
1.547
0.160
1.658
0.166
1.961
0.200
1.628
0.160
Lower Large Intestine Wall
1.739
0.17
1.595
0.161
2.250
0.220
2.368
0.237
4.070
0.400
3.256
0.330
Small Intestine
0.518
0.052
0.5439
0.055
1.195
0.122
1.397
0.141
2.035
0.200
1.628
0.160
Upper Large Intestine Wall
0.962
0.096
0.943
0.096
1.828
0.186
2.0365
0.205
2.442
0.250
1.887
0.190
Kidneys
1.406
0.14
1.088
0.112
1.308
0.129
1.5155
0.154
1.739
0.180
1.443
0.140
Liver
0.3219
0.032
0.3046
0.031
0.394
0.038
0.4262
0.0435
0.481
0.048
0.3626
0.036
Ovaries
0.592
0.058
0.6164
0.061
1.322
0.133
1.5392
0.154
3.330
0.330
2.5900
0.260
Red Marrow
0.1628
0.016
0.1595
0.0161
0.281
0.0277
0.3552
0.0352
0.629
0.063
0.4810
0.050
Testes
0.518
0.051
0.5294
0.053
1.0826
0.110
1.1840
0.122
2.368
0.240
1.628
0.160
Urinary Bladder Wall
11.470
1.1
9.428
0.921
21.090
2.090
23.680
2.368
59.20
6.00
48.1000
4.80
Total Body
0.2405
0.024
0.2176
0.022
0.3656
0.0365
0.4026
0.0410
0.814
0.081
0.6660
0.065
-
HOW SUPPLIED
Kit for the Preparation of Technetium Tc 99m Mertiatide is supplied as a lyophilized powder packaged in vials. Each reaction vial contains 1 mg betiatide, 0.05 mg (minimum) stannous chloride dihydrate (SnCl2∙2H2O), 0.2 mg (maximum) total tin expressed as stannous chloride dihydrate (SnCl2∙2H2O), 40 mg sodium tartrate dihydrate (Na2C4H2O6∙2H2O), and 20 mg lactose monohydrate.
The pH of the reconstituted drug is between 5.0 and 6.0. No bacteriostatic preservative is present.
Packages containing 5 reaction vials (NDC: 45567-0655-1) are available.
-
INSTRUCTIONS FOR THE PREPARATION OF TECHNETIUM TC 99M MERTIATIDE
Note: Read complete directions thoroughly before starting preparation procedure.
Procedural Precautions and Notes
- 1.
Solutions of sodium pertechnetate Tc 99m which contain oxidizing agents (i.e., sodium hypochlorite or hydrogen peroxide) should not be used.
NOTE: Do not use Tc 99m eluate more than 6 hours after its elution from the generator. - 2. All transfers and vial stopper entries must be done using aseptic technique.
- 3. The water bath used for heating the contents of the reaction vial must be at a continuous rolling boil during the heating step of the preparation procedure. The vial should be in direct contact with the rolling boil water of the bath, and the level of the bath must be at least even with the level of the contents of the vial.
- 4. The temperature of a lead incubation shield should be allowed to reach the temperature of the water bath before incubating the reaction vial. The shield should be designed so that water flows through the interior of the shield.
Note 1: Wear waterproof gloves during the entire preparation procedure and during subsequent patient dose withdrawals from the reaction vial.
Note 2: Make all transfers of sodium pertechnetate Tc 99m solution during the preparation procedure with an adequately shielded syringe.
Note 3: Keep the radioactive preparation in the lead shield described below (with cap in place) during the useful life of the radioactive preparation. Maintain adequate shielding during the life of the product and use a shielded, sterile syringe for withdrawing and injecting the preparation.
Procedure for the Preparation of Technetium Tc 99m Mertiatide
- 1. Prepare a rolling boil water bath containing a vial shield with openings cut in it to allow the water to circulate through the shield. The openings should be oriented to prevent radiation leakage.
- 2. Place the reaction vial in a lead dispensing shield fitted with a lid and with a minimum wall thickness of 1/8 inch.
- 3.
Swab the rubber stopper of the reaction vial with an appropriate antiseptic. Insert a filter-containing venting needle (provided) through the vial stopper. Inject 4 to 10 milliliters of sodium pertechnetate Tc 99m solution containing 740 megabecquerels (20 mCi) to 3.70 gigabecquerels (100 mCi) into the vial. If required, use nonbacteriostatic normal saline to dilute the sodium pertechnetate Tc 99m solution to the desired concentration prior to addition to the vial.
NOTE: Make sure the water bath is at boiling temperature before adding sodium pertechnetate Tc 99m to the reaction vial. - 4.
Immediately following the addition of sodium pertechnetate Tc 99m solution to the reaction vial, withdraw the syringe plunger to a volume of 2 mL, thus removing 2 mL of argon gas and adding 2 mL of filtered air to the vial. The air is required to oxidize excess stannous ion. Remove both needles from the vial.
NOTE: The addition of 2 mL air is required to prevent the progressive formation of technetium Tc 99m labeled impurities. - 5. Invert the reaction vial several times to obtain complete mixing.
- 6.
Immediately transfer the reaction vial to the water bath. Place it inside the lead shield which has been equilibrated to the temperature of the boiling water bath. Allow the reaction vial to incubate for 10 minutes.
NOTE: The reaction vial MUST be placed in the boiling water bath within 5 minutes of the addition of sodium pertechnetate Tc 99m solution. - 7. Remove the reaction vial from the boiling water bath and place in the lead dispensing shield. Allow the contents of the vial to cool for approximately 15 minutes to reach body temperature. Using proper shielding, the vial contents should be visually inspected. The solution should be clear and free of particulate matter. If not, the preparation should not be used.
- 8. Assay the reaction vial using a suitable radioactivity calibration system. Record the date, time, total technetium Tc 99m activity, volume, and technetium Tc 99m concentration on the radioassay information label and affix the label to the lead dispensing shield.
- 9. The radiochemical purity of the reconstituted solution must be checked prior to administration to the patient. If the radiochemical purity is less than 90%, the product must not be used.
- 10. Store the reaction vial containing the technetium Tc 99m mertiatide at room temperature (15° to 30°C) until use. The technetium Tc 99m mertiatide preparation must be used within six hours of preparation.
- 1.
Solutions of sodium pertechnetate Tc 99m which contain oxidizing agents (i.e., sodium hypochlorite or hydrogen peroxide) should not be used.
-
RECOMMENDED METHOD FOR DETERMINATION OF RADIO CHEMICAL PURITY OF KIT FOR THE PREPARATION OF TECHNETIUM TC99M MERTIATIDE
Required Materials
Waters Sep-Pak™ C18 Cartridges, Part #51910,
200 proof ethanol
0.9% Sodium Chloride Injection, USP
0.001N hydrochloric acid1
1:1 ethanol/saline solution2
Disposable syringes:
10 mL, no needle required
1 mL, with needle
Disposable culture tubes or vials, minimum 15 mL capacity
Ion chamber for measurement of radioactive samples.
- 1 May be prepared by diluting 1 mL of 0.10N hydrochloric acid to 100 mL with Water for Injection, USP, or by other appropriate dilution of more concentrated hydrochloric acid. For example, 0.1 mL of 36% (~11.6N) hydrochloric acid diluted to a total volume of 1,150 mL.
- 2 Prepared by mixing equal volumes of the 200 proof ethanol and 0.9% Sodium Chloride Injection, USP.
Preparation of Sep-Pak Cartridge
- 1. Using a 10 mL syringe, push 10 mL of 200 proof ethanol through the Sep-Pak cartridge. Discard the eluate.
- 2. Similarly, flush the cartridge with 10 mL of the 0.001N hydrochloric acid. Discard the eluate.
- 3. Drain the cartridge by pushing 5 mL of air through the cartridge with the syringe. Discard the eluate.
Sample Analysis
- 1. Apply 0.1 mL of the technetium Tc 99m mertiatide preparation to the head of the cartridge through the longer end of the cartridge using a 1 mL syringe with needle. Note: The cartridge and all solutions eluted from it will be radioactive after this step.
- 2. With a disposable 10 mL syringe, slowly push 10 mL of 0.001N hydrochloric acid through the cartridge. Collect this fraction in a culture tube or vial for counting.
- 3. Similarly, elute the cartridge with 10 mL of the 1:1 ethanol/saline solution. Be sure that this solution is pushed through the cartridge slowly so that the elution occurs in a drop-wise manner. Collect this 10 mL fraction in a second culture tube or vial for counting.
- 4. Place the Sep-Pak cartridge in a third culture tube or vial for counting.
Counting
- 1. Assay the activity of the first sample elution in an ion chamber. This fraction contains the hydrophilic impurities (free pertechnetate, technetium tartrate, etc.) and a fraction of reduced-hydrolyzed technetium.
- 2. Assay the activity of the second elution. This fraction contains the technetium Tc 99m mertiatide complex.
- 3. Assay the activity of the Sep-Pak cartridge in the third culture tube or vial. This component contains the remaining reduced-hydrolyzed technetium and non-elutable impurities.
Calculations
1. Percent technetium Tc 99m mertiatide =
Activity of 2nd (ethanol/saline) fraction
× 100%
Total activity of all three fractions
2. Percent hydrophilic impurities =
Activity of 1st (0.001N HCl acid) fraction
× 100%
Total activity of all three fractions
3. Percent non-elutable impurities =
Activity remaining on Sep-Pak cartridge
× 100%
Total activity of all three fractions
This reagent kit for the preparation of a radiopharmaceutical is approved for use by persons licensed pursuant to Section 120.547, Code of Massachusetts Regulation 105, or under equivalent license of the U.S. Nuclear Regulatory Commission or an Agreement State.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
KIT FOR THE PREPARATION OF TECHNETIUM TC99M MERTIATIDE
betiatide injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45567-0655 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Betiatide (UNII: 9NV2SR34P8) (Betiatide - UNII:9NV2SR34P8) Betiatide 1 mg Inactive Ingredients Ingredient Name Strength Stannous Chloride (UNII: 1BQV3749L5) 0.05 mg Stannous Chloride Anhydrous (UNII: R30H55TN67) 0.2 mg Sodium Tartrate (UNII: QTO9JB4MDD) 40 mg Lactose Monohydrate (UNII: EWQ57Q8I5X) 20 mg Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45567-0655-1 5 in 1 CARTON; Type 0: Not a Combination Product 09/02/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208994 09/02/2019 Labeler - Pharmalucence, Inc. (139261648)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.