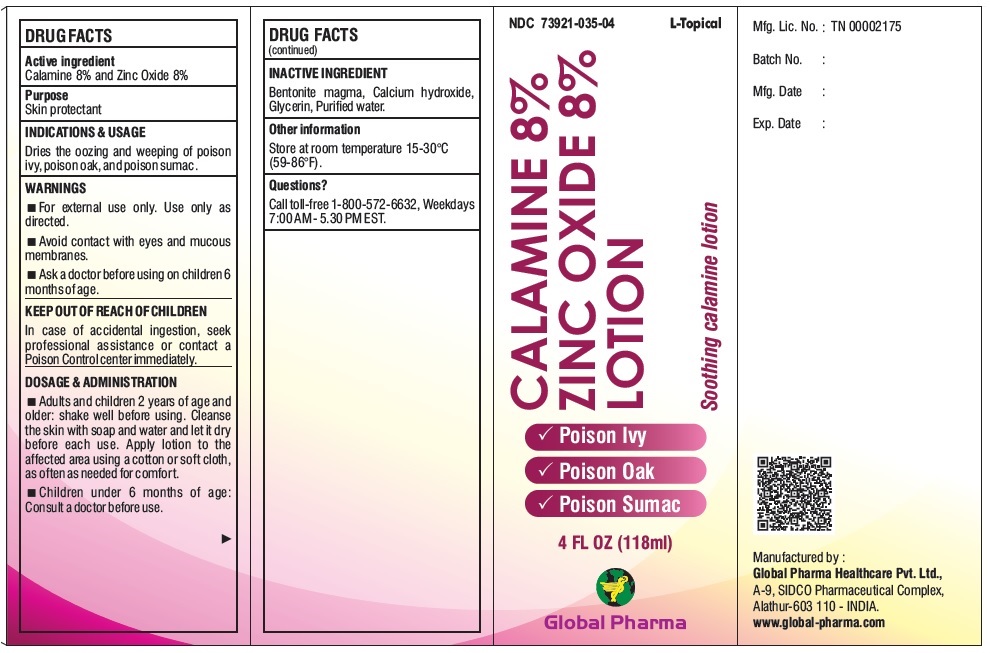
L-Topical CALAMINE 8% ZINC OXIDE 8% LOTION
L-Topical CALAMINE 8% ZINC OXIDE 8% by
Drug Labeling and Warnings
L-Topical CALAMINE 8% ZINC OXIDE 8% by is a Otc medication manufactured, distributed, or labeled by GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
L-TOPICAL CALAMINE 8% ZINC OXIDE 8%- calamine, zinc oxide lotion
GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
L-Topical CALAMINE 8% ZINC OXIDE 8% LOTION
WARNINGS
For external use only. Use only as directed.
Avoid contact with eyes and mucous membranes.
Ask a doctor before using on children 6 months of age.
DOSAGE & ADMINISTRATION
Adults and children 2 years of age and older: shake well before using. Cleanse the skin with soap and water and let it dry before each use. Apply lotion to the affected area using a cotton or soft cloth, as often as needed for comfort.
Children under 6 months of age: Consult a doctor before use.
| L-TOPICAL CALAMINE 8% ZINC OXIDE 8%
calamine, zinc oxide lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED (860186917) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED | 860186917 | manufacture(73921-035) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.