Hair Cream by Shenzhen Yikai Electronic Technology Co., Ltd. 006-01下架
Hair Cream by
Drug Labeling and Warnings
Hair Cream by is a Otc medication manufactured, distributed, or labeled by Shenzhen Yikai Electronic Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
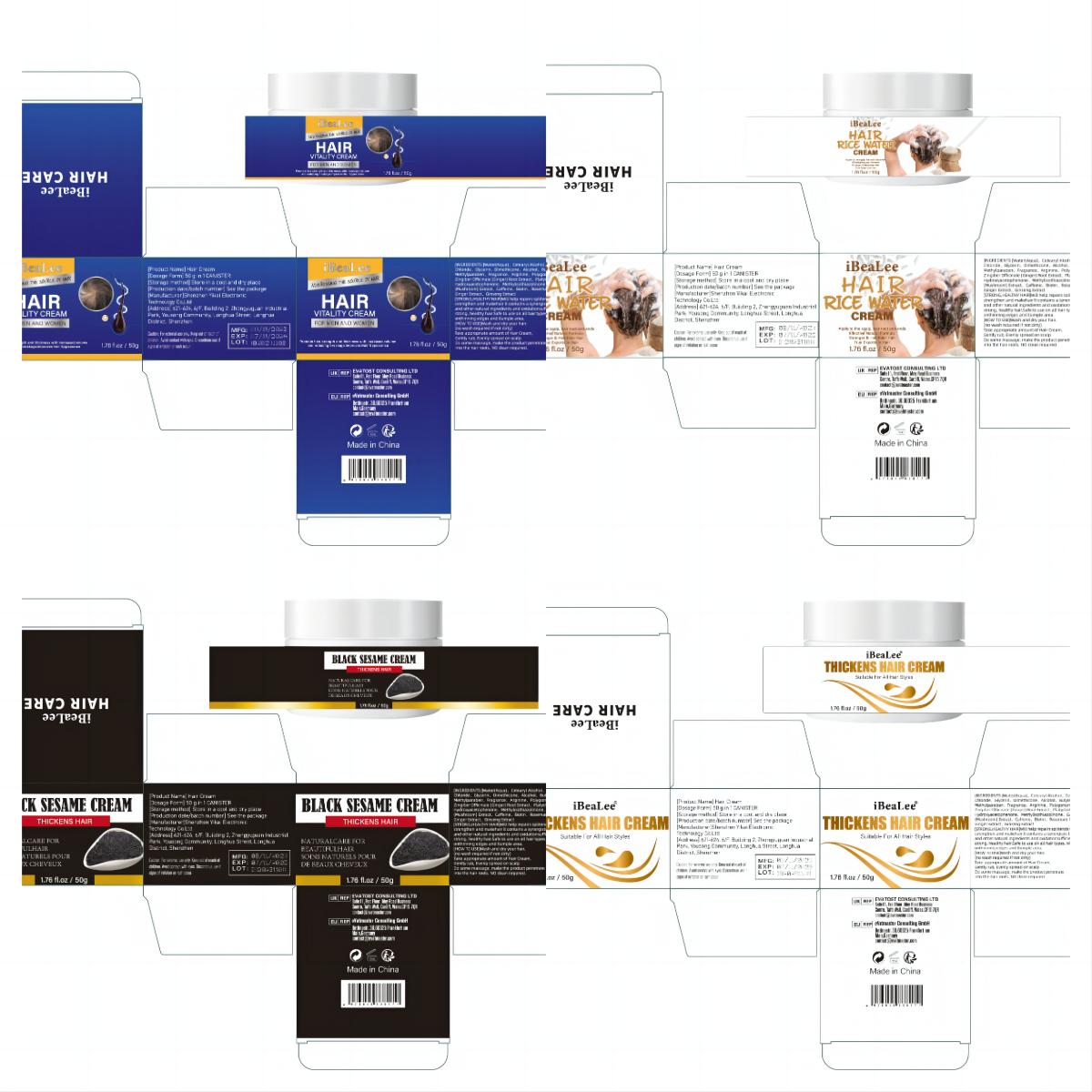
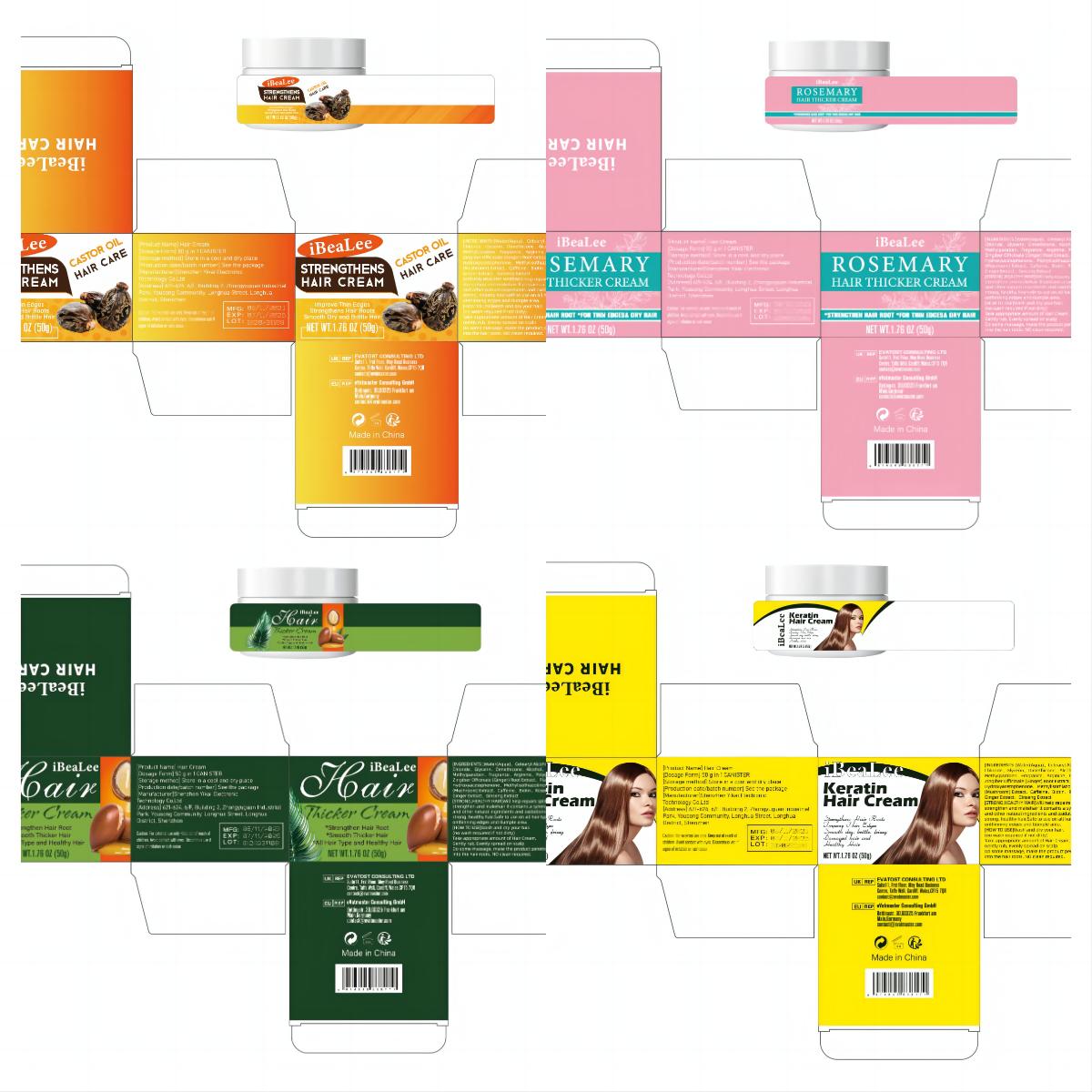
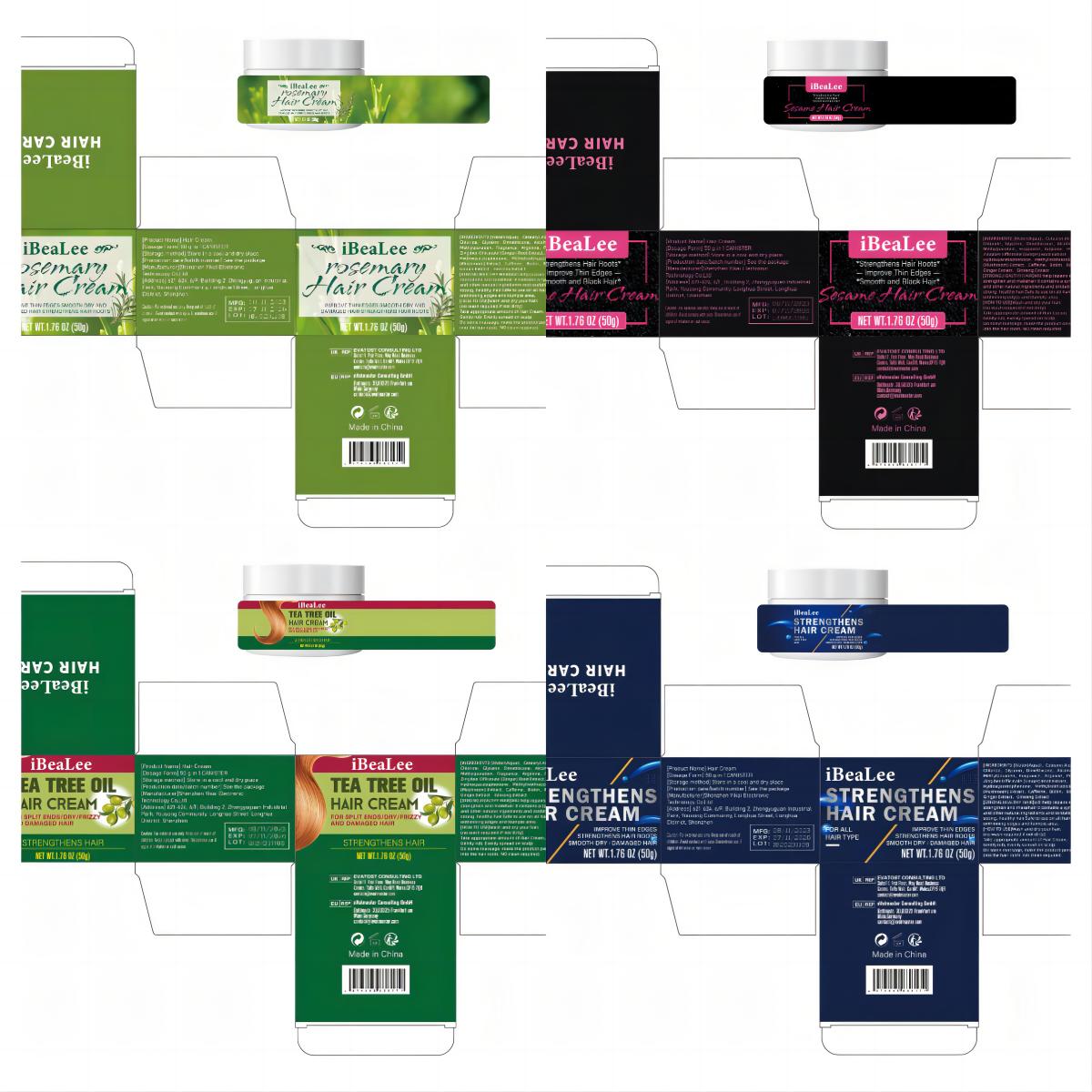
HAIR CREAM- hair cream cream
Shenzhen Yikai Electronic Technology Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
006-01下架
Use
[STRONG,HEALTHY HAIR]Will help repairs splitends/dry/damaged hair,
strengthen and makehair lt contains a synergistic blendof essentialoils
and other natural ingredients and oxidationsufficiently to bring about
strong, healthy hair.Safe to use on all hair types. Works wonders
onthinning edges and tlumple area.
Warnings
For external use only. Keep out of reach of
children. Avoid contact with eyes. Discontinue use if
signs of iritation or rash occur.
Directions
[HOW TO USE]Wash and dry your hair.
(no wash required if not dirty)
Take appropriate amount of Hair Cream,
Gently rub, Evenly spread on scalp
Do some massage, make the product penetrate
into the hair roots. NO clean required.
Inactive ingredients
Water(Aqua), Cetearyl Alcohol, Ceteartrimonium Chloride, Glycerin, Dimethicone, Alcohol, Butylene Glycol, Methylparaben, Fragrance, Arginine, Polygonum Multiflorum Extract, Zingiber Officinale (Ginger) Root Extract, Platycladus Orientalis Extract, Hydroxyacetophenone, Methylisothiazolinone, Ganoderma Atrum (Mushroom) Extract, Caffeine, Rosemary Oil, Castor Oil, Ginger Extract, Ginseng Extrac
| HAIR CREAM
hair cream cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Shenzhen Yikai Electronic Technology Co., Ltd. (700426808) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Yikai Electronic Technology Co., Ltd. | 700426808 | label(83809-006) , manufacture(83809-006) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.