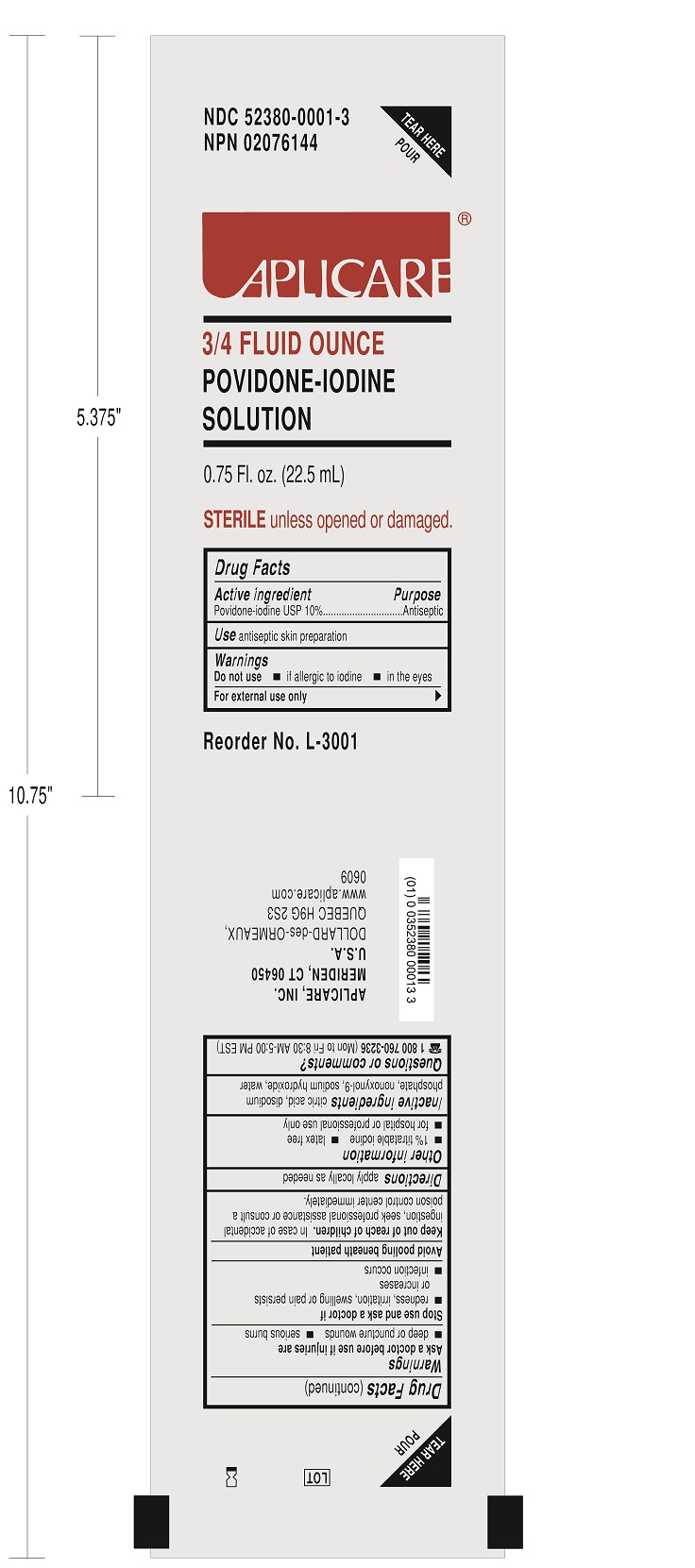
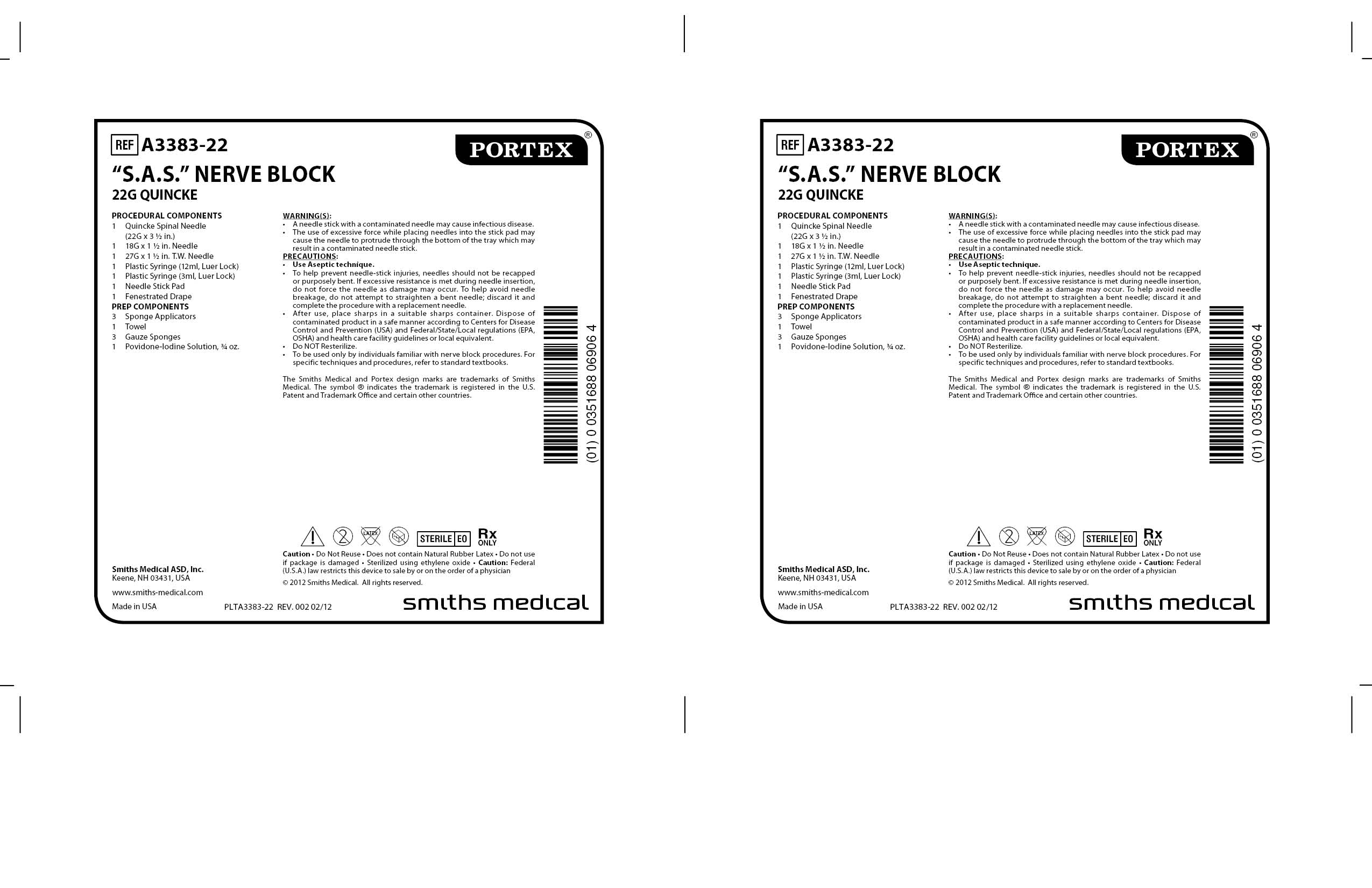
A3383-22 S.A.S. NERVE BLOCK 22G QUINCKE by Smiths Medical ASD, Inc. / Aplicare, Inc.
A3383-22 S.A.S. NERVE BLOCK 22G QUINCKE by
Drug Labeling and Warnings
A3383-22 S.A.S. NERVE BLOCK 22G QUINCKE by is a Other medication manufactured, distributed, or labeled by Smiths Medical ASD, Inc., Aplicare, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
A3383-22 S.A.S. NERVE BLOCK 22G QUINCKE- anesthesia conduction kit
Smiths Medical ASD, Inc.
----------
Do not use
-if allergic to iodine
-in the eyes
For external use only
Ask a doctor before use if injuries are
-deep or puncture wounds
-serious burns
Stop use and ask a doctor if
-redness, irritation, swelling or pain persists or increases
-infection occurs
Avoid pooling beneath patient
Avoid excessive heat. Store at room temperature.
| A3383-22 S.A.S. NERVE BLOCK 22G QUINCKE
anesthesia conduction kit kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare, Inc. | 081054904 | manufacture | |
Revised: 2/2020
Document Id: 95493055-bff8-4ad2-9643-379a2146e165
Set id: 0b627282-0bfe-4726-ab1b-a3f05596f0e1
Version: 3
Effective Time: 20200213