LEVOLEUCOVORIN injection, powder, lyophilized, for solution
levoleucovorin by
Drug Labeling and Warnings
levoleucovorin by is a Prescription medication manufactured, distributed, or labeled by Actavis Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LEVOLEUCOVORIN FOR INJECTION safely and effectively. See full prescribing information for LEVOLEUCOVORIN FOR INJECTION.
LEVOLEUCOVORIN for injection, for intravenous use
Initial U.S. Approval: 1952 (d,l-leucovorin), 2008 (levoleucovorin)INDICATIONS AND USAGE
Levoleucovorin for Injection is a folate analog indicated for:
- Rescue after high-dose methotrexate therapy in osteosarcoma.
- Diminishing the toxicity and counteracting the effects of impaired methotrexate elimination and of inadvertent overdosage of folic acid antagonists.
Limitations of Use
Levoleucovorin for Injection is not approved for pernicious anemia and megaloblastic anemias. Improper use may cause a hematologic remission while neurologic manifestations continue to progress. (1.1)
DOSAGE AND ADMINISTRATION
Do not administer intrathecally. (2.1)
Levoleucovorin for Injection is dosed at one-half the usual dose of racemic d,l-leucovorin. (2.1)
Levoleucovorin for Injection Rescue After High-Dose Methotrexate Therapy
Levoleucovorin for Injection rescue recommendations are based on a methotrexate dose of 12 grams/m2 administered by intravenous infusion over 4 hours. Levoleucovorin for Injection rescue at a dose of 7.5 mg (approximately 5 mg/m2) every 6 hours for 10 doses starts 24 hours after the beginning of the methotrexate infusion. Determine serum creatinine and methotrexate levels at least once daily. Continue Levoleucovorin for Injection administration, hydration, and urinary alkalinization (pH of 7.0 or greater) until the methotrexate level is below 5 x 10-8 M (0.05 micromolar). The Levoleucovorin for Injection dose may need to be adjusted. (2.3)
DOSAGE FORMS AND STRENGTHS
Levoleucovorin for Injection: Each 50 mg single-dose vial of Levoleucovorin for Injection contains a sterile lyophilized powder consisting of 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin) and 50 mg mannitol. (3, 11, 16) It is intended for intravenous administration after reconstitution with 5.3 mL of sterile 0.9% Sodium Chloride Injection, USP. (2.6, 11)
CONTRAINDICATIONS
Levoleucovorin for Injection is contraindicated for patients who have had previous allergic reactions attributed to folic acid or folinic acid. (4)
WARNINGS AND PRECAUTIONS
Due to Ca++ content, no more than 16 mL (160 mg) of levoleucovorin solution should be injected intravenously per minute. (5.1)
Levoleucovorin for Injection enhances the toxicity of fluorouracil. (5.2, 7)
Concomitant use of d,l-leucovorin with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in HIV patients was associated with increased rates of treatment failure in a placebo-controlled study. (5.3)
ADVERSE REACTIONS
Allergic reactions were reported in patients receiving Levoleucovorin for Injection. (6.3)
Vomiting (38%), stomatitis (38%) and nausea (19%) were reported in patients receiving Levoleucovorin for Injection as rescue after high-dose methotrexate therapy. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Actavis at 1-800-432-8534 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Levoleucovorin for Injection may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible patients. (7)
Revised: 12/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Administration Guidelines
2.2 Co-administration of Levoleucovorin for Injection with other agents
2.3 Levoleucovorin for Injection Rescue After High-Dose Methotrexate Therapy
2.4 Dosing Recommendations for Inadvertent Methotrexate Overdosage
2.6 Reconstitution and Infusion Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Rate of Administration
5.2 Potential for Enhanced Toxicity with 5-Fluorouracil
5.3 Potential for Interaction with Trimethoprim-Sulfamethoxazole
6 ADVERSE REACTIONS
6.1 Clinical Studies in High-Dose Methotrexate Therapy
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 High-Dose Methotrexate Therapy
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
- Levoleucovorin for Injection is a folate analog.
- Levoleucovorin for Injection rescue is indicated after high-dose methotrexate therapy in osteosarcoma.
- Levoleucovorin for Injection is also indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosage of folic acid antagonists.
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Guidelines
Levoleucovorin for Injection is dosed at one-half the usual dose of racemic d,l-leucovorin.
Levoleucovorin for Injection is indicated for intravenous administration only. Do not administer intrathecally.
2.2 Co-administration of Levoleucovorin for Injection with other agents
Due to the risk of precipitation, do not co-administer Levoleucovorin for Injection with other agents in the same admixture.
2.3 Levoleucovorin for Injection Rescue After High-Dose Methotrexate Therapy
The recommendations for Levoleucovorin for Injection rescue are based on a methotrexate dose of 12 grams/m2 administered by intravenous infusion over 4 hours (see methotrexate package insert for full prescribing information). Levoleucovorin for Injection rescue at a dose of 7.5 mg (approximately 5 mg/m2) every 6 hours for 10 doses starts 24 hours after the beginning of the methotrexate infusion.
Serum creatinine and methotrexate levels should be determined at least once daily. Levoleucovorin for Injection administration, hydration, and urinary alkalinization (pH of 7.0 or greater) should be continued until the methotrexate level is below 5 x 10-8 M (0.05 micromolar). The Levoleucovorin for Injection dose should be adjusted or rescue extended based on the following guidelines.
Table 1 Guidelines for Levoleucovorin for Injection Dosage and Administration Clinical Situation Laboratory Findings Levoleucovorin for Injection Dosage and Duration Normal Methotrexate Elimination Serum methotrexate level approximately 10 micromolar at 24 hours after administration, 1 micromolar at 48 hours, and less than 0.2 micromolar at 72 hours 7.5 mg intravenously q 6 hours for 60 hours (10 doses starting at 24 hours after start of methotrexate infusion). Delayed Late Methotrexate Elimination Serum methotrexate level remaining above 0.2 micromolar at 72 hours, and more than 0.05 micromolar at 96 hours after administration. Continue 7.5 mg intravenously q 6 hours, until methotrexate level is less than 0.05 micromolar. Delayed Early Methotrexate Elimination and/or Evidence of Acute Renal Injury Serum methotrexate level of 50 micromolar or more at 24 hours, or 5 micromolar or more at 48 hours after administration, OR; a 100% or greater increase in serum creatinine level at 24 hours after methotrexate administration (e.g., an increase from 0.5 mg/dL to a level of 1 mg/dL or more). 75 mg intravenously q 3 hours until methotrexate level is less than 1 micromolar; then 7.5 mg intravenously q 3 hours until methotrexate level is less than 0.05 micromolar. Patients who experience delayed early methotrexate elimination are likely to develop reversible renal failure. In addition to appropriate Levoleucovorin for Injection therapy, these patients require continuing hydration and urinary alkalinization, and close monitoring of fluid and electrolyte status, until the serum methotrexate level has fallen to below 0.05 micromolar and the renal failure has resolved.
Some patients will have abnormalities in methotrexate elimination or renal function following methotrexate administration, which are significant but less severe than the abnormalities described in the table above. These abnormalities may or may not be associated with significant clinical toxicity. If significant clinical toxicity is observed, Levoleucovorin for Injection rescue should be extended for an additional 24 hours (total of 14 doses over 84 hours) in subsequent courses of therapy. The possibility that the patient is taking other medications which interact with methotrexate (e.g., medications which may interfere with methotrexate elimination or binding to serum albumin) should always be reconsidered when laboratory abnormalities or clinical toxicities are observed.
Delayed methotrexate excretion may be caused by accumulation in a third space fluid collection (i.e., ascites, pleural effusion), renal insufficiency, or inadequate hydration. Under such circumstances, higher doses of Levoleucovorin for Injection or prolonged administration may be indicated.
Although Levoleucovorin for Injection may ameliorate the hematologic toxicity associated with high-dose methotrexate, Levoleucovorin for Injection has no effect on other established toxicities of methotrexate such as the nephrotoxicity resulting from drug and/or metabolite precipitation in the kidney.
2.4 Dosing Recommendations for Inadvertent Methotrexate Overdosage
Levoleucovorin for Injection rescue should begin as soon as possible after an inadvertent overdosage and within 24 hours of methotrexate administration when there is delayed excretion. As the time interval between antifolate administration [e.g., methotrexate] and Levoleucovorin for Injection rescue increases, Levoleucovorin for Injection’s effectiveness in counteracting toxicity may decrease. Levoleucovorin for Injection 7.5 mg (approximately 5 mg/m2) should be administered intravenously every 6 hours until the serum methotrexate level is less than 10-8 M.
Serum creatinine and methotrexate levels should be determined at 24 hour intervals. If the 24 hour serum creatinine has increased 50% over baseline or if the 24 hour methotrexate level is greater than 5 x 10-6 M or the 48 hour level is greater than 9 x 10-7 M, the dose of Levoleucovorin for Injection should be increased to 50 mg/m2 intravenously every 3 hours until the methotrexate level is less than 10-8 M. Hydration (3 L/day) and urinary alkalinization with NaHCO3 should be employed concomitantly. The bicarbonate dose should be adjusted to maintain the urine pH at 7.0 or greater.
2.6 Reconstitution and Infusion Instructions
Levoleucovorin for Injection
- Prior to intravenous injection, the 50 mg vial of Levoleucovorin for Injection is reconstituted with 5.3 mL of 0.9% Sodium Chloride Injection, USP to yield a levoleucovorin concentration of 10 mg per mL. Reconstitution with Sodium Chloride solutions with preservatives (e.g. benzyl alcohol) has not been studied. The use of solutions other than 0.9% Sodium Chloride Injection, USP is not recommended.
- The reconstituted 10 mg per mL levoleucovorin contains no preservative. Observe strict aseptic technique during reconstitution of the drug product.
- Saline reconstituted levoleucovorin solutions may be further diluted, immediately, to concentrations of 0.5 mg/mL to 5 mg/mL in 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. Initial reconstitution or further dilution using 0.9% Sodium Chloride Injection, USP may be held at room temperature for not more than a total of 12 hours. Dilutions in 5% Dextrose Injection, USP may be held at room temperature for not more than 4 hours.
- Visually inspect the reconstituted solution for particulate matter and discoloration, prior to administration. CAUTION: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if cloudiness or precipitate is observed.
- No more than 16 mL of reconstituted solutions (160 mg of levoleucovorin) should be injected intravenously per minute, because of the calcium content of the levoleucovorin solution.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Rate of Administration
Because of the Ca++ content of the levoleucovorin solution, no more than 16 mL (160 mg of levoleucovorin) should be injected intravenously per minute.
5.2 Potential for Enhanced Toxicity with 5-Fluorouracil
Levoleucovorin for Injection enhances the toxicity of 5-fluorouracil. Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients receiving weekly d,l-leucovorin and 5-fluorouracil. Gastrointestinal toxicities (particularly stomatitis and diarrhea) are observed more commonly and may be of greater severity and of prolonged duration. Seizures and/or syncope have been reported rarely in cancer patients receiving d,l-leucovorin, usually in association with fluoropyrimidine administration, and most commonly in those with CNS metastases or other predisposing factors. However, a causal relationship has not been established.
5.3 Potential for Interaction with Trimethoprim-Sulfamethoxazole
The concomitant use of d,l-leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection was associated with increased rates of treatment failure and morbidity in a placebo-controlled study.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies in High-Dose Methotrexate Therapy
Since clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The following table presents the frequency of adverse reactions which occurred during the administration of 58 courses of high-dose methotrexate 12 grams/m2 followed by Levoleucovorin for Injection rescue for osteosarcoma in 16 patients age 6 to 21. Most patients received Levoleucovorin for Injection 7.5 mg every 6 hours for 60 hours or longer beginning 24 hours after completion of methotrexate.
Table 2 Adverse Reactions with High-Dose Methotrexate Therapy Body System/Adverse Reactions Number (%) of Patients with Adverse Reactions Number (%) of Courses with Adverse Reactions (N = 16) (N = 58) All Grade 3+ All Grade 3+ Gastrointestinal Stomatitis 6 (37.5) 1 (6.3) 10 (17.2) 1 (1.7) Vomiting 6 (37.5) 0 14 (24.1) 0 Nausea 3 (18.8) 0 3 (5.2) 0 Diarrhea 1 (6.3) 0 1 (1.7) 0 Dyspepsia 1 (6.3) 0 1 (1.7) 0 Typhlitis 1 (6.3) 1 (6.3) 1 (1.7) 1 (1.7) Respiratory Dyspnea 1 (6.3) 0 1 (1.7) 0 Skin and Appendages Dermatitis 1 (6.3) 0 1 (1.7) 0 Other Confusion 1 (6.3) 0 1 (1.7) 0 Neuropathy 1 (6.3) 0 1 (1.7) 0 Renal function abnormal 1 (6.3) 0 3 (5.2) 0 Taste perversion 1 (6.3) 0 1 (1.7) 0 Total number of patients 9 (56.3) 2 (12.5) Total number of courses 25 (43.1) 2 (3.4) The incidence of adverse reactions may be underestimated because not all patients were fully evaluable for toxicity for all cycles in the clinical trials. Leukopenia and thrombocytopenia were observed, but could not be attributed to high-dose methotrexate with Levoleucovorin for Injection rescue because patients were receiving other myelosuppressive chemotherapy.
6.3 Postmarketing Experience
Since adverse reactions from spontaneous reports are provided voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. Spontaneously reported adverse reactions collected by the WHO Collaborating Center for International Drug Monitoring in Uppsala Sweden have yielded seven cases where levoleucovorin was administered with a regimen of methotrexate. The events were dyspnea, pruritus, rash, temperature change and rigors. For 217 adverse reactions (108 reports) where levoleucovorin was a suspected or interacting medication, there were 40 occurrences of “possible allergic reactions.”
In an analysis where calcium levoleucovorin was reported as the primary suspect drug and fluorouracil (FU) was reported as a concomitant medication, possible allergic reactions were reported among 47 cases (67 events).
-
7 DRUG INTERACTIONS
Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible children. It is not known whether folinic acid has the same effects.
However, both folic and folinic acids share some common metabolic pathways. Caution should be taken when taking folinic acid in combination with anticonvulsant drugs.
Preliminary human studies have shown that small quantities of systemically administered leucovorin enter the CSF, primarily as its major metabolite, 5-methyltetrahydrofolate (5-MTHFA). In humans, the CSF levels of 5-MTHFA remain 1 to 3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration.
Levoleucovorin for Injection increases the toxicity of 5-fluorouracil [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
Animal reproduction studies have not been conducted with Levoleucovorin for Injection. It is not known whether Levoleucovorin for Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Levoleucovorin for Injection should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Levoleucovorin for Injection, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Levoleucovorin is the levo isomeric form of racemic d,l-leucovorin, present as the calcium salt. Levoleucovorin is the pharmacologically active isomer of leucovorin [(6-S)-leucovorin].
Levoleucovorin for Injection contains levoleucovorin calcium, which is one of several active, chemically reduced derivatives of folic acid. It is useful as antidote to the inhibition of dihydrofolate reductase by methotrexate. This compound has the chemical designation calcium (6S)-N-{4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl] amino]benzoyl}-L-glutamate pentahydrate. The molecular weight is 601.6 and the structural formula is:
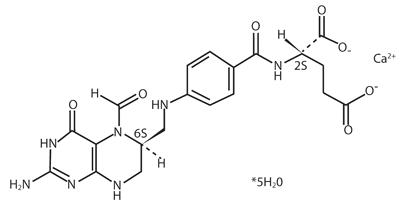
Its molecular formula is: C20H21CaN7O7 5 H2O.
Levoleucovorin for Injection is supplied as a sterile lyophilized powder consisting of 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin) and 50 mg mannitol per 50 mg vial. Sodium hydroxide and/or hydrochloric acid are used to adjust the pH during manufacture. It is intended for intravenous administration after reconstitution with 5.3 mL of sterile 0.9% Sodium Chloride Injection, USP [see Dosage and Administration (2.6)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.1.1 Levoleucovorin effects during high-dose methotrexate therapy
Levoleucovorin is the pharmacologically active isomer of 5-formyl tetrahydrofolic acid. Levoleucovorin does not require reduction by the enzyme dihydrofolate reductase in order to participate in reactions utilizing folates as a source of “one-carbon” moieties. Administration of levoleucovorin can counteract the therapeutic and toxic effects of folic acid antagonists such as methotrexate, which act by inhibiting dihydrofolate reductase.
12.2 Pharmacodynamics
Levoleucovorin is actively and passively transported across cell membranes. In vivo, levoleucovorin is converted to 5-methyltetrahydrofolic acid (5-methyl-THF), the primary circulating form of active reduced folate. Levoleucovorin and 5-methyl-THF are polyglutamated intracellularly by the enzyme folylpolyglutamate synthetase. Folylpolyglutamates are active and participate in biochemical pathways that require reduced folate.
12.3 Pharmacokinetics
The pharmacokinetics of levoleucovorin after intravenous administration of a 15 mg dose was studied in healthy male volunteers. After rapid intravenous administration, serum total tetrahydrofolate (total-THF) concentrations reached a mean peak of 1722 ng/mL. Serum (6S)-5-methyl-5,6,7,8-tetrahydrofolate concentrations reached a mean peak of 275 ng/mL and the mean time to peak was 0.9 hours. The mean terminal half-life for total-THF and (6S)-5-methyl-5,6,7,8-tetrahydrofolate was 5.1 and 6.8 hours, respectively.
A pharmacokinetic study was conducted in 40 healthy subjects who received a single intravenous dose of either Levoleucovorin for Injection (200 mg/m2) or racemic d,l-leucovorin (400 mg/m2), each administered as a 2-hour infusion in a crossover design. Results indicate that the 90% confidence interval for the geometric mean ratios for both AUC0-inf and Cmax were within the standard limit of 80 to 125% for both l-leucovorin and l-5-methyl-THF. Therefore, the exposure to l-leucovorin and 5-methyl-THF (AUC0-inf and Cmax) was comparable whether it was administered as Levoleucovorin for Injection or as d,l-leucovorin. The geometric mean AUC0-inf values for levoleucovorin were 30719 ng.h/mL and 31296 ng.h/mL for Levoleucovorin for Injection and d,l-leucovorin, respectively. The geometric mean Cmax values for levoleucovorin were 10895 ng/mL and 11301 ng/mL for Levoleucovorin for Injection and d,l-leucovorin, respectively. The geometric mean AUC0-inf values for 5-methyl-THF were 52105 ng.h/mL and 50137 ng.h/mL for Levoleucovorin for Injection and d,l-leucovorin, respectively. The geometric mean Cmax values for 5-methyl-THF were 4930 ng/mL and 4658 ng/mL for Levoleucovorin for Injection and d,l-leucovorin, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to evaluate the potential of levoleucovorin for carcinogenesis, mutagenesis and impairment of fertility.
13.2 Animal Toxicology and/or Pharmacology
The acute intravenous LD50 values in adult mice and rats were 575 mg/kg (1725 mg/m2) and 378 mg/kg (2268 mg/m2), respectively. Signs of sedation, tremors, reduced motor activity, prostration, labored breathing, and/or convulsion were observed in these studies. Anticipated human dose for each administration is approximately 5 mg/m2 for high-dose methotrexate therapy which represents a 3-log safety margin.
-
14 CLINICAL STUDIES
14.1 High-Dose Methotrexate Therapy
The safety and efficacy of Levoleucovorin for Injection rescue following high-dose methotrexate were evaluated in 16 patients age 6 to 21 who received 58 courses of therapy for osteogenic sarcoma. High-dose methotrexate was one component of several different combination chemotherapy regimens evaluated across several trials. Methotrexate 12 g/m2 intravenously over 4 hours was administered to 13 patients, who received Levoleucovorin for Injection 7.5 mg every 6 hours for 60 hours or longer beginning 24 hours after completion of methotrexate. Three patients received methotrexate 12.5 g/m2 intravenously over 6 hours, followed by Levoleucovorin for Injection 7.5 mg every 3 hours for 18 doses beginning 12 hours after completion of methotrexate. The mean number of Levoleucovorin for Injection doses per course was 18.2 and the mean total dose per course was 350 mg. The efficacy of Levoleucovorin for Injection rescue following high-dose methotrexate was based on the adverse reaction profile [see Adverse Reactions (6)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each 50 mg single-dose vial of Levoleucovorin for Injection contains a sterile lyophilized powder consisting of 64 mg levoleucovorin calcium pentahydrate (equivalent to 50 mg levoleucovorin) and 50 mg mannitol.
50 mg vial of freeze-dried powder – NDC: 45963-762-57.
Store at 25°C (77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light. Store in carton until contents are used.
Sterile, Nonpyrogenic, Preservative-free.
The vial stopper is not made with natural rubber latex.
Made in Italy
Distributed by:
Actavis Pharma, Inc.
Parsippany, NJ 07054 USARevised – December 2016
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEVOLEUCOVORIN
levoleucovorin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45963-762 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOLEUCOVORIN CALCIUM (UNII: 778XL6VBS8) (LEVOLEUCOVORIN - UNII:990S25980Y) LEVOLEUCOVORIN 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 10 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45963-762-57 1 in 1 CARTON 02/14/2017 1 5.3 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206516 02/14/2017 Labeler - Actavis Pharma, Inc. (119723554)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
