Curae by Curae Pharma360 Inc. / Naari Pte. Limited Curae™
Curae by
Drug Labeling and Warnings
Curae by is a Otc medication manufactured, distributed, or labeled by Curae Pharma360 Inc., Naari Pte. Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CURAE- levonorgestrel tablet
Curae Pharma360 Inc.
----------
Curae™
Use
for women to reduce chance of pregnancy after unprotected sex
(if a contraceptive failed or if you did not use birth control)
Warnings
Sexually transmitted diseases (STDs) alert
This product does not protect against HIV/AIDS or other STDs
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
Directions
- take as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it, the better it will work.
- If you vomit within 2 hours of taking the medication, call a healthcare professional to find out if you should repeat the dose
Other information
- read the instructions, warnings, and enclosed product leaflet before use
- do not use if carton is open or blister seal is broken or missing
- store at 20° to 25°C (68° to 77°F)
Inactive ingredients
colloidal silicon dioxide, FD&C Yellow No.6 aluminum lake, lactose monohydrate, magnesium stearate, and pregelatinized starch
Manufactured for:
Curae Pharma360 Inc.
San Francisco, CA 94105
NDC: 73358-241-01
Product made in Germany
Issued: March 2023
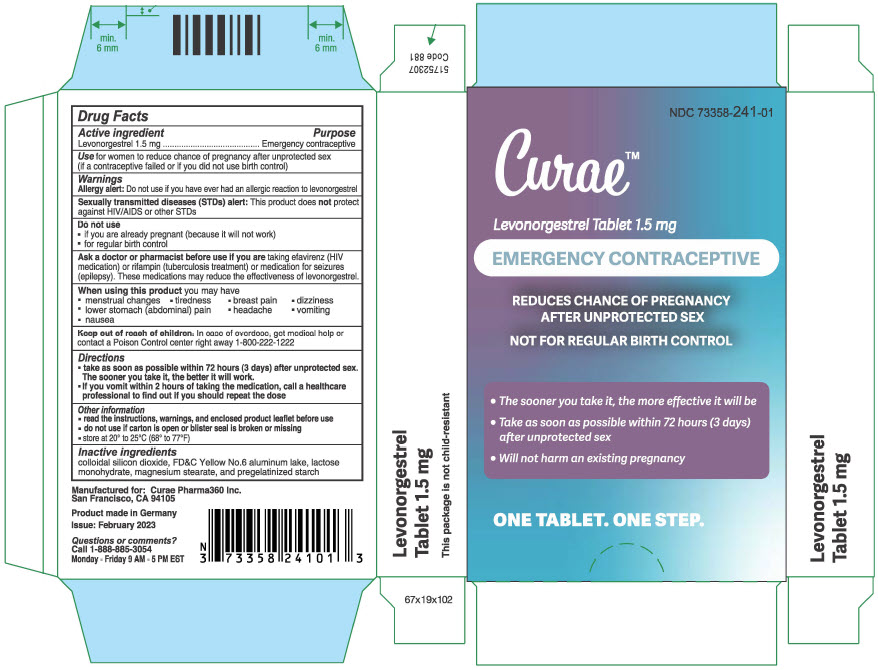
PRINCIPAL DISPLAY PANEL - 1.5 mg Tablet Blister Pack Carton
NDC: 73358-241-01
Curae™
Levonorgestrel Tablet 1.5 mg
EMERGENCY CONTRACEPTIVE
REDUCES CHANCE OF PREGNANCY
AFTER UNPROTECTED SEX
NOT FOR REGULAR BIRTH CONTROL
- The sooner you take it, the more effective it will be
-
Take as soon as possible within 72 hours (3 days)
after unprotected sex - Will not harm an existing pregnancy
ONE TABLET. ONE STEP.
| CURAE
levonorgestrel tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Curae Pharma360 Inc. (080719852) |
| Registrant - Naari Pte. Limited (659345996) |
Trademark Results [Curae]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CURAE 97790160 not registered Live/Pending |
Curae Pharma360, Inc. 2023-02-10 |
 CURAE 88136098 not registered Live/Pending |
Provider Web Capital Funding, LLC 2018-09-28 |
 CURAE 88136094 5745252 Live/Registered |
Provider Web Capital Funding, LLC 2018-09-28 |
 CURAE 88031645 not registered Live/Pending |
Curae, LLC 2018-07-10 |
 CURAE 74292761 1891572 Dead/Cancelled |
SAGIT S.P.A. 1992-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.