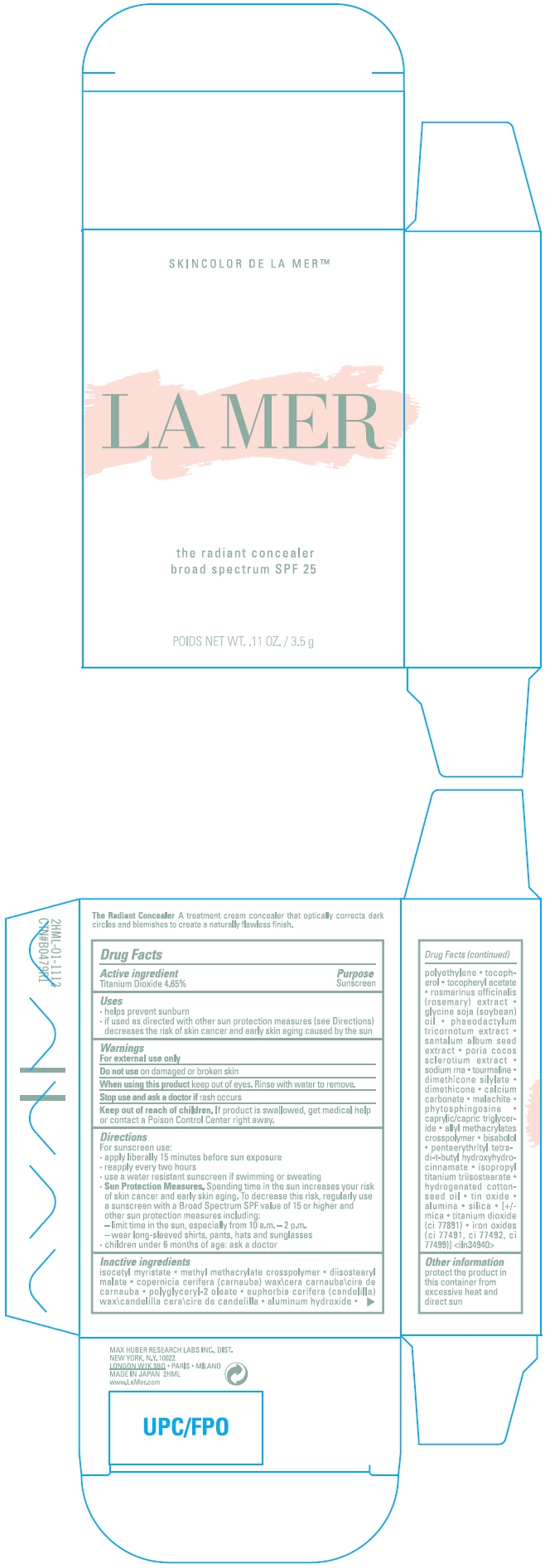
THE RADIANT CONCEALER BROAD SPECTRUM SPF 25- titanium dioxide cream
THE RADIANT CONCEALER BROAD SPECTRUM SPF 25 by
Drug Labeling and Warnings
THE RADIANT CONCEALER BROAD SPECTRUM SPF 25 by is a Otc medication manufactured, distributed, or labeled by MAX HUBER RESEARCH LAB INC, ELGC K.K., ESTEE LAUDER COMPANY, THE, ESTEE LAUDER COSMETICS, LTD, ESTEE LAUDER COSMETICS, LTD., ESTEE LAUDER N.V., LEN-RON MANUFACTURING DIVISION OF ARAMIS INC, NORTHTEC INC, NORTHTEC LLC, PADC 1, WHITMAN LABORATORIES, LTD., Aveda Corporation, Pennsylvania Logistics Center (PALC), Toshiki Pigment Company Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- – limit time in the sun, especially from 10 a.m. – 2 p.m.
- – wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
isocetyl myristate methyl methacrylate crosspolymer diisostearyl malate copernicia cerifera (carnauba) wax\cera carnauba\cire de carnauba polyglyceryl-2 oleate euphorbia cerifera (candelilla) wax\candelilla cera\cire de candelilla aluminum hydroxide polyethylene tocopherol tocopheryl acetate rosmarinus officinalis (rosemary) extract glycine soja (soybean) oil phaeodactylum tricornotum extract santalum album seed extract poria cocos sclerotium extract sodium rna tourmaline dimethicone silylate dimethicone calcium carbonate malachite phytosphingosine caprylic/capric triglyceride allyl methacrylates crosspolymer bisabolol pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate isopropyl titanium triisostearate hydrogenated cottonseed oil tin oxide alumina silica [+/-mica titanium dioxide (ci 77891) iron oxides (ci 77491, ci 77492, ci 77499)] <iln34940>
- Other information
- PRINCIPAL DISPLAY PANEL - 3.5 g Container Carton
-
INGREDIENTS AND APPEARANCE
THE RADIANT CONCEALER BROAD SPECTRUM SPF 25
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65966-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.5 g in 100 g Inactive Ingredients Ingredient Name Strength isocetyl myristate (UNII: 69AX3BRR5N) diisostearyl malate (UNII: QBS8A3XZGQ) carnauba wax (UNII: R12CBM0EIZ) candelilla wax (UNII: WL0328HX19) aluminum hydroxide (UNII: 5QB0T2IUN0) high density polyethylene (UNII: UG00KM4WR7) tocopherol (UNII: R0ZB2556P8) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) rosemary (UNII: IJ67X351P9) soybean oil (UNII: 241ATL177A) phaeodactylum tricornutum (UNII: Y5W63E7HLV) santalum album seed (UNII: 7RZT0U509Y) dimethicone (UNII: 92RU3N3Y1O) calcium carbonate (UNII: H0G9379FGK) phytosphingosine (UNII: GIN46U9Q2Q) medium-chain triglycerides (UNII: C9H2L21V7U) levomenol (UNII: 24WE03BX2T) pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) (UNII: 255PIF62MS) isopropyl titanium triisostearate (UNII: 949E3KBJ1I) hydrogenated cottonseed oil (UNII: Z82Y2C65EA) stannic oxide (UNII: KM7N50LOS6) aluminum oxide (UNII: LMI26O6933) silicon dioxide (UNII: ETJ7Z6XBU4) mica (UNII: V8A1AW0880) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65966-015-01 1 in 1 CARTON 1 3.5 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/01/2014 Labeler - MAX HUBER RESEARCH LAB INC (926708694) Establishment Name Address ID/FEI Business Operations ELGC K.K. 712808195 RELABEL(65966-015) , REPACK(65966-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(65966-015) , REPACK(65966-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(65966-015) , REPACK(65966-015) Establishment Name Address ID/FEI Business Operations NORTHTEC LLC 959338336 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(65966-015) , REPACK(65966-015) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations Pennsylvania Logistics Center (PALC) 078364654 REPACK(65966-015) , RELABEL(65966-015) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(65966-015) Establishment Name Address ID/FEI Business Operations Toshiki Pigment Company Limited 716140285 MANUFACTURE(65966-015)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.