TERUFLEX BLOOD BAG SYSTEM WITH BLOOD SAMPLING ARM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL (AS-5) RED CELL PRESERVATIVE (anticoagulant citrate phosphate dextrose- cpd and as-5 red cell preservative kit
TERUFLEX Blood Bag System with Blood Sampling Arm Anticoagulant Citrate Phosphate Dextrose (CPD) AND OPTISOL (AS-5) Red Cell Preservative by
Drug Labeling and Warnings
TERUFLEX Blood Bag System with Blood Sampling Arm Anticoagulant Citrate Phosphate Dextrose (CPD) AND OPTISOL (AS-5) Red Cell Preservative by is a Prescription medication manufactured, distributed, or labeled by Terumo Corporation, Terumo Corp. - Fujinomiya Factory. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
INDICATIONS & USAGE
Issued 8/96
TERUFLEX® BLOOD BAG SYSTEM With Blood Sampling Arm® CPD/OPTISOL® SOLUTION
Read these instructions carefully before use.
INSTRUCTIONS FOR BLOOD COLLECTION: Use aseptic technique
Materials Needed:
VENOJECT® ll Tube Holder (code P-1316R)
VENOJECT® ll Multi-Sample Luer Adapter (code MN *2000)
VENOJECT® ll Plastic Blood Collection Tubes (or equivalent glass or plastic evacuated blood collection tube)
1. Confirm that all numbered tubing of each blood bag unit contains segment numbers.
2. Make a loose knot in donor tubing below "Y" unless alternate methods are used to seal tubing.
3. Clamp donor tubing.
4. Suspend primary bag as far as possible below the donor's arm.
5. Apply blood pressure cuff or tourniquet to donor's arm. Disinfect site of phlebotomy. If blood pressure cuff is used, inflate cuff to approximately 60 mmHg.
6. Remove needle protector and perform phlebotomy. Remove clamp to permit blood flow into primary bag.
CAUTION Do not touch needle after removing the needle protector.
7. Appropriately secure donor tubing to donor's arm.
8. MIX BLOOD WITH ANTICOAGULANT AT SEVERAL INTERVALS DURING COLLECTION.
9. Collect labeled volume of blood (+/–10%).
10. Tighten knot firmly after collection. Clamp between knot and "Y". Sever donor tubing between the knot and clamp. Alternate methods may be used to seal tubing.
CAUTION Do not use the dielectric tube sealer while the needle is connected to the donor's body.
Anytime before Step #13 below, sever donor tubing between the two seals.
Collect blood samples as follows:
a) Connect VENOJECT II Multi-Sample Luer Adapter to VENOJECT II Tube Holder (Fig. 1).
b) Remove covers and connect multi-sample luer adapter to female luer at end of donor sampling tubing (Fig. 2).
c) Snap CLIKTIP in donor sampling tubing to open blood pathway.
Insert Fig. 1 here

Insert Fig. 2 here
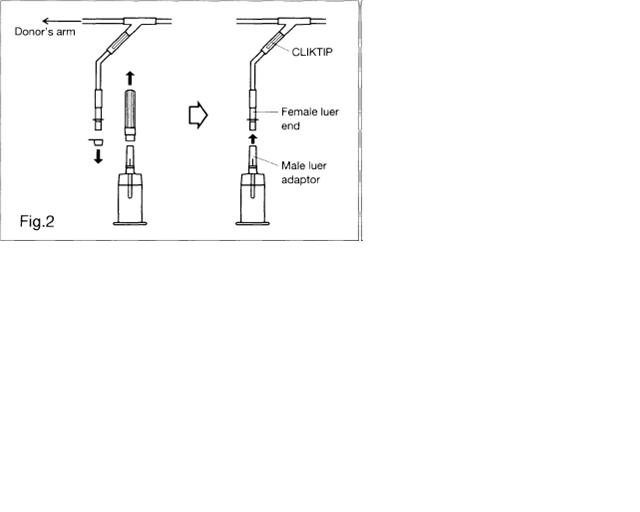
d) Insert blood collection tube (VENOJECT II or equivalent) firmly into VENOJECT II Tube Holder : when full, remove sample tube from holder. Repeat to collect additional samples.
11. Reapply clamp to donor tubing between phlebotomy needle and "Y"; release pressure on donor's arm and remove needle.
CAUTION Discard tubing/phlebotomy needle unit according to institutional procedures.
12. Immediately after collection, invert bag several times to assure blood and anticoagulant are well mixed.
13. Strip blood from the donor tubing into bag, mix well, and allow tubing to refill. Seal on or near X marks on donor tubing to provide numbered aliquots of anticoagulated blood for testing.
14. The time of addition of OPTISOL Solution may vary depending on the processing option selected. Add the solution under one of the following conditions.
a) After removal of plasma from freshly collected blood.
b) Within 8 hours of blood collection if components are prepared.
c) Within 72 hours of collection if blood is refrigerated immediately following collection.
15. Centrifuge the unit to separate red cells from plasma.
16. Snap CLlKTIP (inline closure device) of primary collection bag and transfer plasma into satellite bag. Clamp transfer tubing of satellite bag.
17. Snap CLlKTIP of OPTlSOL Solution bag and drain contents into primary bag containing red blood cells. Seal tubing of primary bag in two places, and cut between seals and separate from satellite bag(s).
NOTE: For TERUFLEX double bags, seal OPTISOL Solution bag tubing in two places and cut between seals. Discard OPTISOL Solution container.
18. Invert the red cell- OPTISOL mixture several times to insure the final AS-5 red cell product is well suspended.
19. Store AS-5 Red Blood Cells between 1-6°C.
20. Infuse AS-5 Red Blood Cells within 42 days of collection.
For further processing, use standard component processing techniques.
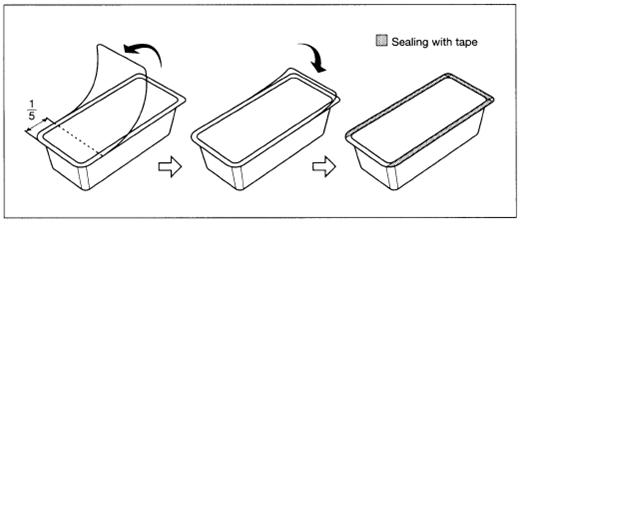
To open blister package, peel cover film back four fifths of its length.
After opening, unused bags may be stored for 30 days by returning cover film to original position and sealing with tape to prevent possible loss of moisture.
Insert Fig. 3 here
CAUTIONS
THE PACKET OF AGELESS CONTAINED IN THIS PACKAGE ABSORBS OXYGEN AND GENERATES HEAT ON REMOVAL AND SHOULD BE HANDLED WITH CARE.
DISPOSE WITH PACKET IN TRAY.
DO NOT DISPOSE WITH WASTES CONTAINING VOLATILE OR FLAMMABLE MATERIALS.
DISCARD AGELESS PACKET WITHOUT OPENING.
TERUMO® TERUMO CORPORATION 44-1, 2-CHOME, HATAGAYA, SHIBUYA-KU, TOKYO, JAPAN
®: Registered Trademark
N-BB-OP-A(SP) 3
-
PRINCIPAL DISPLAY PANEL
Tray/Case Label
TERUFLEX® BLOOD BAG SYSTEM with
BLOOD SAMPLING ARM®
CPD WITH OPTISOL® RED CELL PRESERVATIVE SOLUTION
FOR COLLECTION OF 450mL OF BLOOD
Each unit consists of a collection bag containing 63mL of Anticoagulant
CPD solution, with a satellite bag containing 100mL of OPTlSOL Red
Cell Preservative Solution.
Each 63mL Anticoagulant CPD solution USP contains 1.61g Dextrose
(monohydrate) USP, 1.66g Sodium Citrate (dihydrate) USP, 188mg Citric
Acid (anhydrous) USP, 140mg Monobasic Sodium Phosphate
(monohydrate) USP.
Each 100mL OPTISOL Red Cell Preservative Solution contains 877mg
Sodium Chloride USP, 900mg Dextrose (monohydrate) USP, 525mg
Mannitol USP, 30mg Adenine USP.
STERILE, NON-PYROGENIC FLUID PATH.
DO NOT USE UNLESS ANTICOAGULANT IS CLEAR.
CODE
LOT No.
EXPIRY
UNITS
DONOR NEEDLE 16G x 1 1/2˝ (1.60 x 38mm)
Rx ONLY
RECOMMENDED STORAGE: Room Temperature (15-30°C/59-86°F).
Avoid excessive heat. Protect from freezing.
After opening, unused bags may be stored for 30 days by returning cover film to original
position and sealing with tape to prevent possible loss of moisture.
See Instructions For Blood Collection.
Manufactured by : TERUMO CORPORATION Tokyo, Japan
® : Registered Trademark
Blood Sampling Arm is a trademark of TERUMO CORPORATION.
Rev. 01/03
B-4-G6-A4 4
Place Label here

-
INGREDIENTS AND APPEARANCE
TERUFLEX BLOOD BAG SYSTEM WITH BLOOD SAMPLING ARM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL (AS-5) RED CELL PRESERVATIVE
anticoagulant citrate phosphate dextrose (cpd) and as-5 red cell preservative kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 53877-009 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53877-009-31 24 in 1 CASE 1 1 in 1 BAG Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 63 mL Part 2 1 BAG 100 mL Part 1 of 2 ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD)
anticoagulant citrate phosphate dextrose (cpd) solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 26.3 g in 1000 mL Sodium Phosphate, Monobasic, Unspecified Form (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR, SODIUM CATION - UNII:LYR4M0NH37) Sodium Phosphate, Monobasic, Unspecified Form 2.22 g in 1000 mL Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 25.5 g in 1000 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) Anhydrous Citric Acid 2.99 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 63 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 05/05/2010 Part 2 of 2 OPTISOL RED CELL PRESERVATIVE
as-5 red cell preservative solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) Sodium Chloride 877 mg in 100 mL Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 900 mg in 100 mL Mannitol (UNII: 3OWL53L36A) (Mannitol - UNII:3OWL53L36A) Mannitol 525 mg in 100 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 30 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 100 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 05/05/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 05/05/2010 Labeler - Terumo Corporation (690543319) Establishment Name Address ID/FEI Business Operations Terumo Corp. - Fujinomiya Factory 695214015 MANUFACTURE(53877-009) , STERILIZE(53877-009) , ANALYSIS(53877-009) , LABEL(53877-009)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.