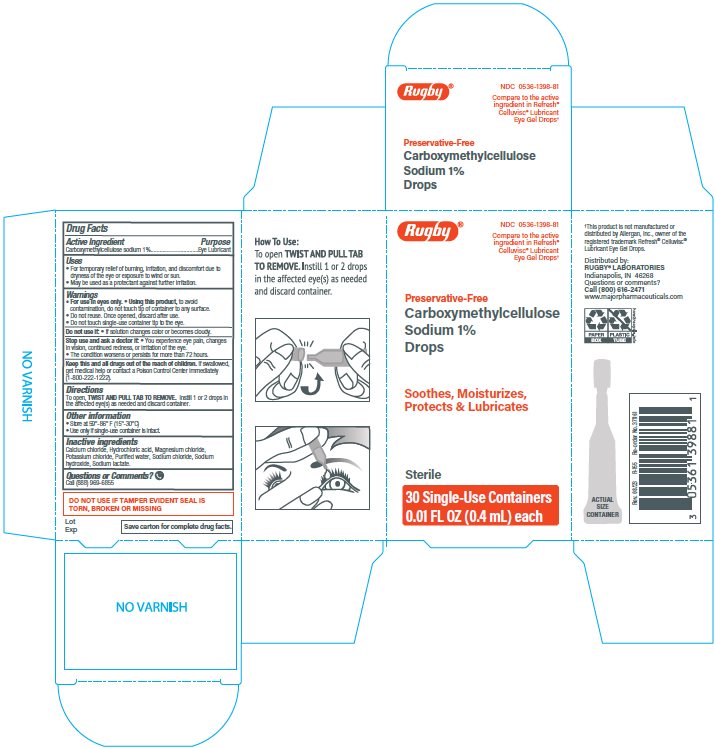
Rugby®Carboxymethylcellulose Sodium 1% Drops
Carboxymethylcellulose Sodium 1% by
Drug Labeling and Warnings
Carboxymethylcellulose Sodium 1% by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARBOXYMETHYLCELLULOSE SODIUM 1%- carboxymethylcellulose sodium liquid
Rugby Laboratories
----------
Rugby®Carboxymethylcellulose Sodium 1% Drops
Uses
- ▪ For the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
- ▪ May be used as a protectant against further irritation.
Warnings
For use in eyes only.
Using this product, to avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard after use.
Do not touch single-use container tip to the eye.
Directions
To open, TWIST AND PULL TAB TO REMOVE. Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
Inactive ingredients
Calcium chloride, Hydrochloric acid, Magnesium chloride, Potassium chloride, Purified water, Sodium chloride, Sodium hydroxide, Sodium lactate.
Do not use
If solution changes color or becomes cloudy.
IF TAMPER EVIDENT SEAL IS TORN, BROKEN OR MISSING
Save carton for complete drug facts.
*This product is not manufactured or distributed by Allergan, Inc., owner of the registered trademark Refresh® Celluvisc® Lubricant Eye Gel Drops.
Distributed by:
RUGBY LABORATORIES
Indianapolis, IN 46268
Questions or comments?
Call (800) 616-2471
|
Rev. 08/23 |
R-155 |
Re-order No. 371161 |
Principal Display Panel
Rugby®
NDC: 0536-1398-81
Compare to the active
ingredient in Refresh®
Celluvisc® Lubricant
Eye Gel Drops*
Preservation-Free
Carboxymethylcellulose
Sodium 1%
Drops
Soothes, Moisturizes,
Protects & Lubricates
Sterile
30 Single-Use Containers
0.01 FL OZ (0.4 mL) each
| CARBOXYMETHYLCELLULOSE SODIUM 1%
carboxymethylcellulose sodium liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.